THE PRODUCTS PURCHASED ON THIS WEBSITE ARE INTENDED FOR RESEARCH CHEMICAL USE ONLY. These products are not intended to be used for human or animal consumption and/or ingestion of any kind. These products should not be used as food additives, drugs or household chemicals. These products should only be used by Qualified Professionals.
Bodily introduction into Human and Animals of any kind is strictly forbidden by law.
All the product information on this website is for educational purpose only.
Tesamorelin
Product Usage: THE PRODUCT IS INTENDED AS A RESEARCH PURPOSE ONLY. This designation allows the use of research chemical strictly for in vitro testing and laboratory experimentation only. All product information available on www.peptidesworld.com is for educational purposes only. Bodily introduction of any food, cosmetic and may not be misbranded, misused or mislabeled as a drug, food or cosmetics.
Highlights:
- Increase lean muscle density
- Loss of abdomen fat
- Improved cognition in adults over the age of 60
- Reduced Carotid Intima Media Size (cIMT)
- Adipotide studied as cancer treatment
Tesamorelin, a growth hormone-releasing hormone analogue, decreases visceral adipose tissue in people living with HIV.
Lean muscle increase
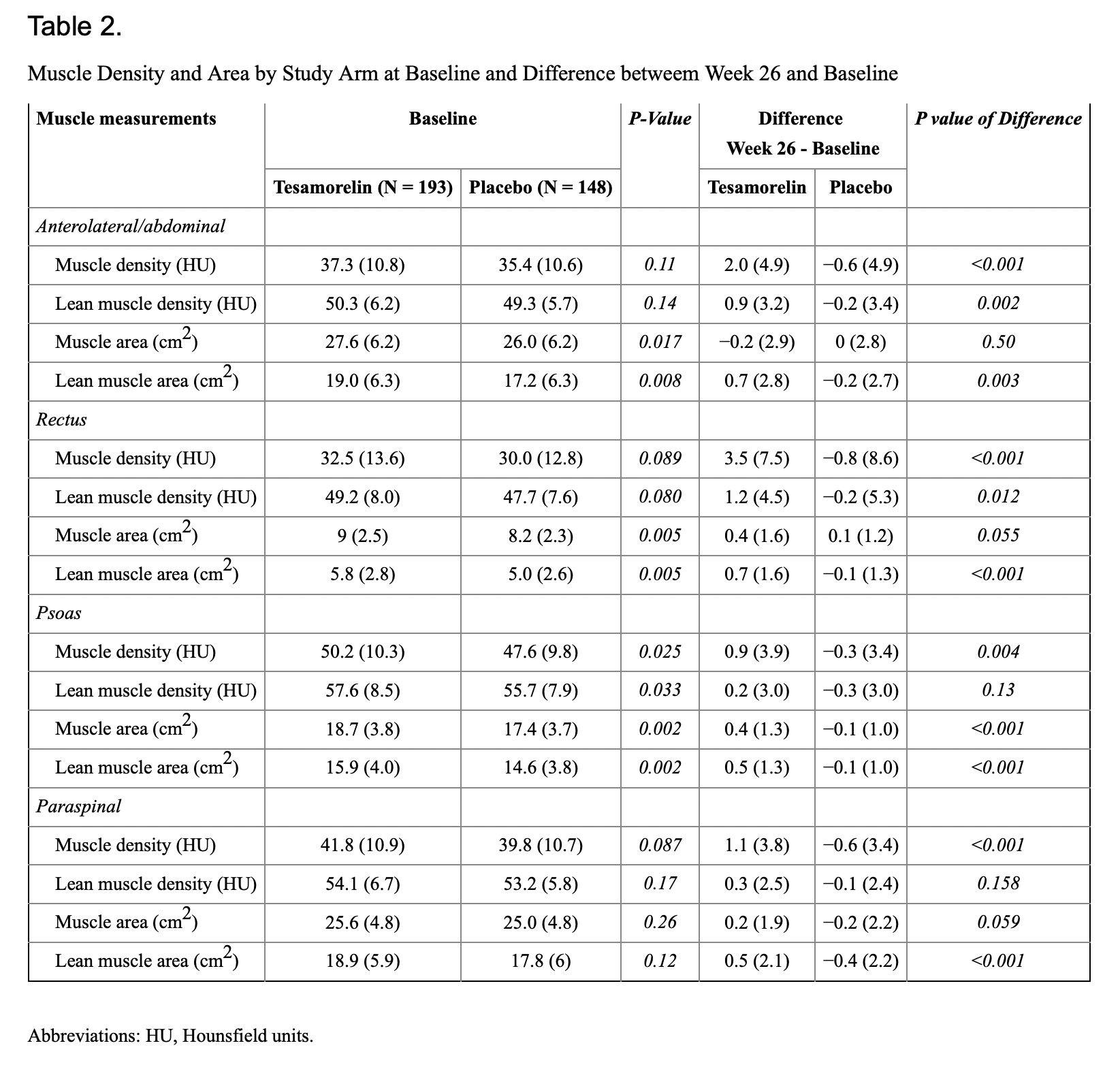
Compared to placebo, 26 weeks of tesamorelin was associated with significantly greater increases of total muscle density (less muscle fat) within all four truncal muscle groups (rectus abdominis, anterolateral abdominal, psoas major and paraspinal muscle groups, p < 0.005); Table 2. The largest net increase was observed within the rectus abdominis (3.5 HU) of the tesamorelin arm. Although increases were also seen in the lean component of muscle density, only differences in anterolateral abdominal muscles were significantly greater in the tesamorelin vs placebo arms (p<0.005) [1]
Multivariate analyses adjusted for baseline values + treatment arm, and then one additional variable (change in VAT or IGF-1) are shown in Table 4. Compared to placebo, tesamorelin resulted in significantly greater increases in muscle density across all total muscle groups and in the lean anterolateral/abdominal and rectus muscle, even after adjusting for differences at baseline. After adjusting for changes in VAT, changes in total muscle density of both the rectus and paraspinal muscles remained significant, while other total and lean muscle groups were attenuated and no longer significant. Lastly, adjustment for changes in IGF-1 slightly attenuated effects, but statistically significant effects persisted across most of the total (anterolateral/abdominal, rectus, paraspinal) and the anterolateral/abdominal lean muscle component. [1]
Tesamorelin & reduction in abdomen fat
In a preliminary study of HIV-infected patients with abdominal fat accumulation, tesamorelin administered for 6 months was associated with reductions in visceral fat and additionally with modest reductions in liver fat. Further studies are needed to determine the clinical importance and long-term consequences of these findings.
Improved cognition in adults over the age of 60
Twenty weeks of GHRH administration had favorable effects on cognition in both adults with MCI and healthy older adults. Longer-duration treatment trials are needed to further examine the therapeutic potential of GHRH administration on brain health during normal aging and “pathological aging.” [3]
Reduced Carotid Intima Media Size (cIMT)
The extent of intervention effects on cIMT progression predicted the degree of CVD risk reduction. This provides a missing link supporting the usefulness of cIMT progression as a surrogate marker for CVD risk in clinical trials. [4]
Tesamorelin reduces cholesterol & Triglyceride
Treatment with tesamorelin resulted in significant decreases in triglycerides (−37 ± 139 vs. 6 ± 112 mg/dl, P < 0.001; treatment effect, −12.3%) and cholesterol to high-density lipoprotein ratio (−0.18 ± 1.00 vs. 0.18 ± 0.94, P < 0.001; treatment effect, −7.2%) vs. placebo. Tesamorelin improved body image [belly appearance distress (P = 0.002)], patient rating of belly profile (P = 0.003), and physician rating of belly profile (P < 0.001). Mean IGF-I increased 108 ± 112 vs.−7 ± 64 ng/ml (P < 0.001 vs. placebo). At wk 52, decreases in VAT [−35 ± 50 cm2 (−17.5 ± 23.3%)], waist circumference (−3.4 ± 6.0 cm), triglycerides (−48 ± 182 mg/dl), cholesterol (−8 ± 38 mg/dl), and non-high-density lipoprotein (−7 ± 38 mg/dl) were maintained (all P < 0.001 vs. original baseline) in the T-T group. Treatment with tesamorelin was generally well tolerated. No clinically meaningful differences were observed between groups in glucose parameters at wk 26 and 52. [5]
- Adrian, S et al. “The Growth Hormone Releasing Hormone Analogue, Tesamorelin, Decreases Muscle Fat and Increases Muscle Area in Adults with HIV.” The Journal of frailty & aging 8,3 (2019): 154-159. doi:10.14283/jfa.2018.45
- Stanley TL, Feldpausch MN, Oh J, Branch KL, Lee H, Torriani M, Grinspoon SK. Effect of tesamorelin on visceral fat and liver fat in HIV-infected patients with abdominal fat accumulation: a randomized clinical trial. JAMA. 2014 Jul 23-30;312(4):380-9. doi: 10.1001/jama.2014.8334. PMID: 25038357; PMCID:
- Baker LD, Barsness SM, Borson S, Merriam GR, Friedman SD, Craft S, Vitiello MV. Effects of growth hormone–releasing hormone on cognitive function in adults with mild cognitive impairment and healthy older adults: results of a controlled trial. Arch Neurol. 2012 Nov;69(11):1420-9. doi: 10.1001/archneurol.2012.1970. PMID: 22869065; PMCID: PMC3764914.
- Peter Willeit, Lena Tschiderer
Carotid Intima-Media Thickness Progression as Surrogate Marker for Cardiovascular Risk, Circulation, Aug 18th 2020, Vol 142 Issue 7, Article download
- Julian Falutz, Jean-Claude Mamputu, Diane Potvin, Graeme Moyle, Graziella Soulban, Helen Loughrey, Christian Marsolais, Ralph Turner, Steven Grinspoon, Effects of Tesamorelin (TH9507), a Growth Hormone-Releasing Factor Analog, in Human Immunodeficiency Virus-Infected Patients with Excess Abdominal Fat: A Pooled Analysis of Two Multicenter, Double-Blind Placebo-Controlled Phase 3 Trials with Safety Extension Data,The Journal of Clinical Endocrinology & Metabolism, Volume 95, Issue 9, 1 September 2010, Pages 4291–4304, https://doi.org/10.1210/jc.2010-0490