PNC-27-5mg
$99.90
PNC-27 has been shown to be an Anti-cancer peptides (ACPs) are a series of short peptides composed of PNC-27, that can inhibit tumor cell proliferation or migration, or suppress the formation of tumor blood vessels, and are less likely to cause drug resistance.
- Buy 5 and save 10%
- Buy 10 and save 15%
THE PRODUCTS PURCHASED ON THIS WEBSITE ARE INTENDED FOR RESEARCH CHEMICAL USE ONLY. These products are not intended to be used for human or animal consumption and/or ingestion of any kind. These products should not be used as food additives, drugs or household chemicals. These products should only be used by Qualified Professionals.
Bodily introduction into Human and Animals of any kind is strictly forbidden by law.
All the product information on this website is for educational purpose only.
Molecular Formula: C188H293N53O44S
Molecular Mass: 4031.7
HIGHLIGHTS:
- PNC-27 a potential Anticancer
- PNC-27 induces Necrosis in Leukemia Cells
- Adverse event
Anticancer peptide PNC-27 adopts an HDM-2-binding conformation and kills cancer cells by binding to HDM-2 in their membranes [1]
The anticancer peptide PNC-27, which contains an HDM-2-binding domain corresponding to residues 12-26 of p53 and a transmembrane-penetrating domain, has been found to kill cancer cells (but not normal cells) by inducing membranolysis. We find that our previously determined 3D structure of the p53 residues of PNC-27 is directly superimposable on the structure for the same residues bound to HDM-2, suggesting that the peptide may target HDM-2 in the membranes of cancer cells. We now find significant levels of HDM-2 in the membranes of a variety of cancer cells but not in the membranes of several untransformed cell lines. In colocalization experiments, we find that PNC-27 binds to cell membrane-bound HDM-2. We further transfected a plasmid expressing full-length HDM-2 with a membrane-localization signal into untransformed MCF-10-2A cells not susceptible to PNC-27 and found that these cells expressing full-length HDM-2 on their cell surface became susceptible to PNC-27. We conclude that PNC-27 targets HDM-2 in the membranes of cancer cells, allowing it to induce membranolysis of these cells selectively.
We have found previously that the peptide PNC-27, containing the HDM-2-binding domain of p53, residues 12-26, attached to a transmembrane-penetrating or membrane residency peptide (MRP) on its carboxyl terminal end, and PNC-28 (p53 residues 17-26-MRP) induce tumor cell necrosis by forming pores in cancer cell membranes but have no effects on a number of untransformed cells, including human stem cells from cord blood (1–4), and eradicate tumors in nude mice (5). The p53-HDM-2 complex results in the catabolism of p53 (6).
In contrast, other studies using HDM-2-binding molecules including p53 peptides attached to membrane-penetrating sequences on their amino terminal ends and small molecules that block the p53-HDM-2 interaction found that these agents induce p53-dependent apoptosis of cancer cells (6–11). However, our observations were consistent with the results from a 2D NMR study on the structure of PNC-27 showing that this peptide adopts a strongly amphipathic alpha-helix-loop-alpha-helix structure (12) observed in membrane-active peptides (13, 14). While this study suggested the basis of their lytic action, it did not explain why these peptides lyse the membranes of cancer cells selectively.
Because these peptides contain sequences that are known to bind to HDM-2 in its amino terminal domain, residues 1-109 (15), we now investigate whether they interact with HDM-2 that may be expressed in the membranes of transformed but not untransformed cells.
Results
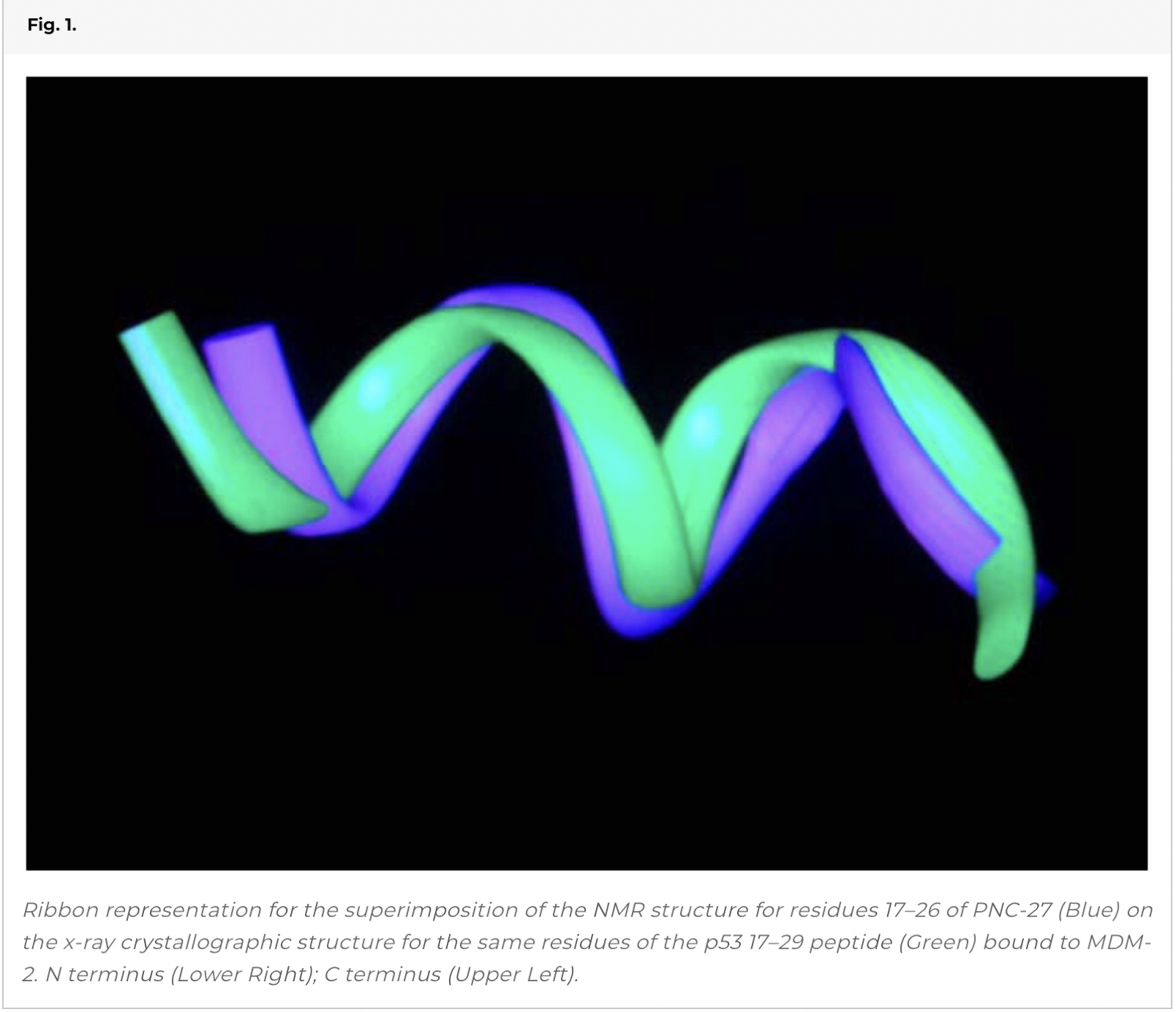
Superposition of the 2D NMR Structures of the HDM-2-Binding Domain of PNC-27 on the X-Ray Structure of the Isolated Peptide Bound to HDM-2.
In our prior 2D NMR determination of the solution structure of PNC-27, we obtained 30 structures that fit the NOE constraints (12). When we superimposed these structures on the x-ray structure, all superimposed closely, giving a range of rms deviations of 1.7–2.5 Å. Fig. 1shows the superposition for the backbone atoms of the structure of lowest rms deviation on the x-ray structure. This agreement occurs despite the fact that the x-ray structure contains a “truncated” peptide beginning at residue 17 and is bound to HDM-2 while PNC-27 contains six additional amino terminal residues (p53 residues 12–16) and Leu 26 attached to the transmembrane-penetrating sequence, not present on the peptide used in the x-ray crystal structure. We conclude that residues 17-26 of PNC-27 fold into a native HDM-2-binding conformation.
Cytotoxicity of PNC-27 to Cancer Cells.
We tested PNC-27 against the cancer cell lines used in this study. As shown in Fig. S1, we found that PNC-27, but not control PNC-29 peptide, is cytotoxic to MIA-PaCa-2 cells, inducing 100% cell death in 90 min and MIA-PaCa-2, TUC-3, and A-2058 cells in a dose-related manner but did not affect untransformed AG13145 primary human fibroblasts. Previously, we found that PNC-27 kills MCF-7 cells by a nonapoptotic (16) mechanism (2).
Blotting for HDM-2 in Cancer Cell Membranes.
To test whether cancer cells express HDM-2 in their cell membranes, we isolated the membrane fractions (confirmed by electron microscopy) and whole cell lysates from several different cancer and untransformed cell lines shown in Fig. 2 and blotted them for HDM-2. On the lower panel for the blots in Fig. 2, it can be seen that all whole cell lysates blot positively for HDM-2. On the upper panel for the blots in Fig. 2, the membrane fraction of each cancer cell line (lanes 4–7) is seen to contain significant levels of HDM-2. In contrast, the three untransformed cell lines (lanes 1–3) were found to have low levels of HDM-2 in their membrane fractions. The percentage of whole cell lysate of HDM-2 present in the membrane fractions of the cell lines is shown in the bar graph (lowermost in Fig. 2). It can be seen that the fractions present in the membranes of the cancer cell lines are fourfold to ninefold increased over those for the untransformed cells. It should be noted that the HDM-2 bands shown in Fig. 2 were the major band at 92kDa. However, several other less prominent bands of lower Mr representing variants of HDM-2 were also observed in the membrane fractions of cancer cells that were absent in the three untransformed cell lines. We are currently investigating the identity of these variant forms. In other control experiments, we blotted the membrane fractions and whole cell lysates of each cell line for p53 and found that none of the membrane fractions contained this protein while it was present in the cell lysates. Our results suggest that HDM-2 is expressed in the membranes of cancer cells but only minimally in those of untransformed cells.
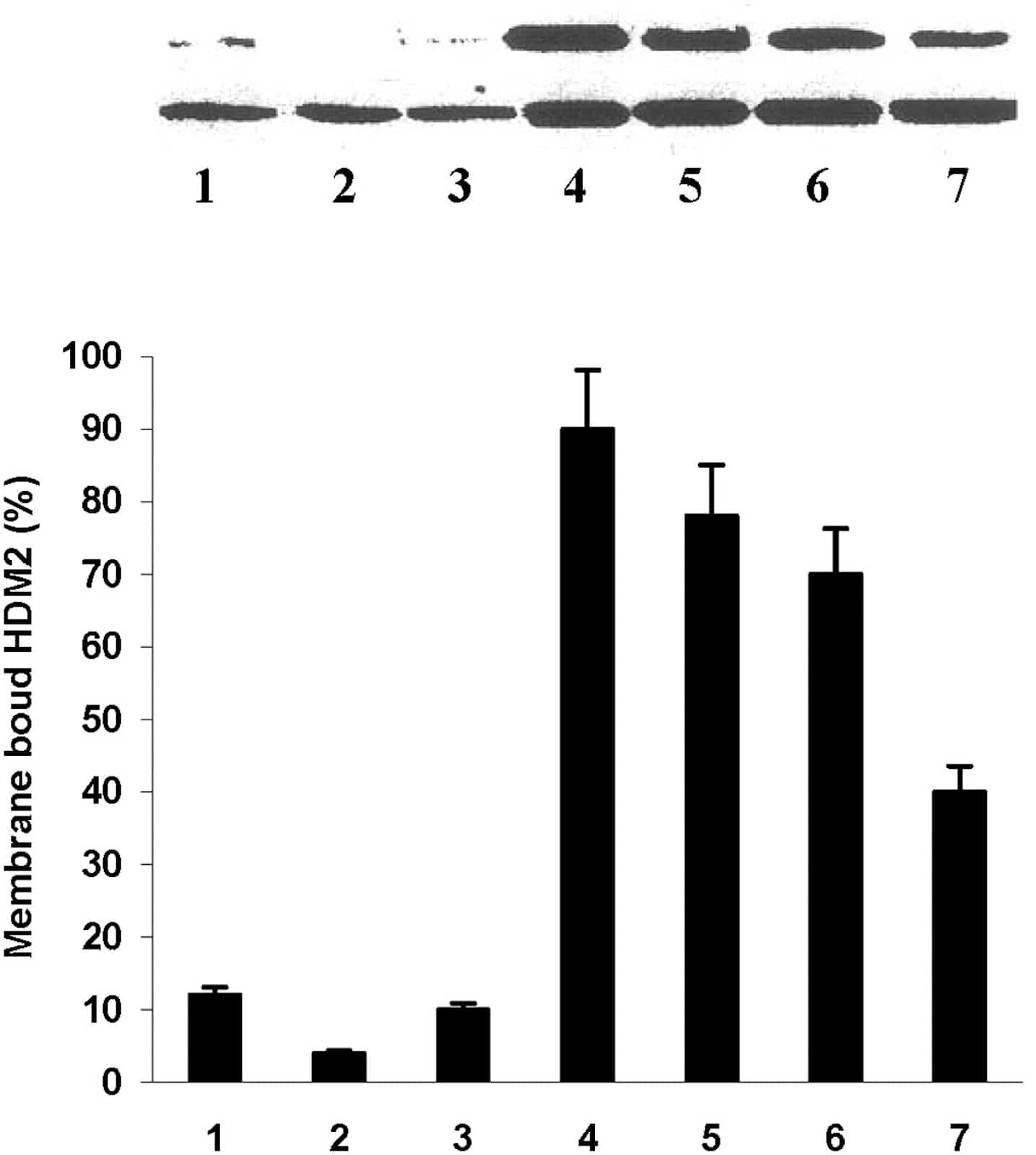
Blots of whole cell lysates (Lower) and membrane fractions (Upper) for H(M)DM-2 in different cell lines as follows: Lane 1, MCF-10-2A; lane 2, BMRPA1; lane 3, AG13145 fibroblasts; lane 4, TUC-3; lane 5, MIA-PaCa-2; lane 6, MCF-7; lane 7, A-2058. The first three cell lines are untransformed; the remainders are different cancer cell lines. Each bar graph shows the percentage of HDM-2 in whole cell lysate that is present in the membrane of each cell line listed above. The numbers on the X axis of the bar graphs correspond to the lane numbers shown in the blots.
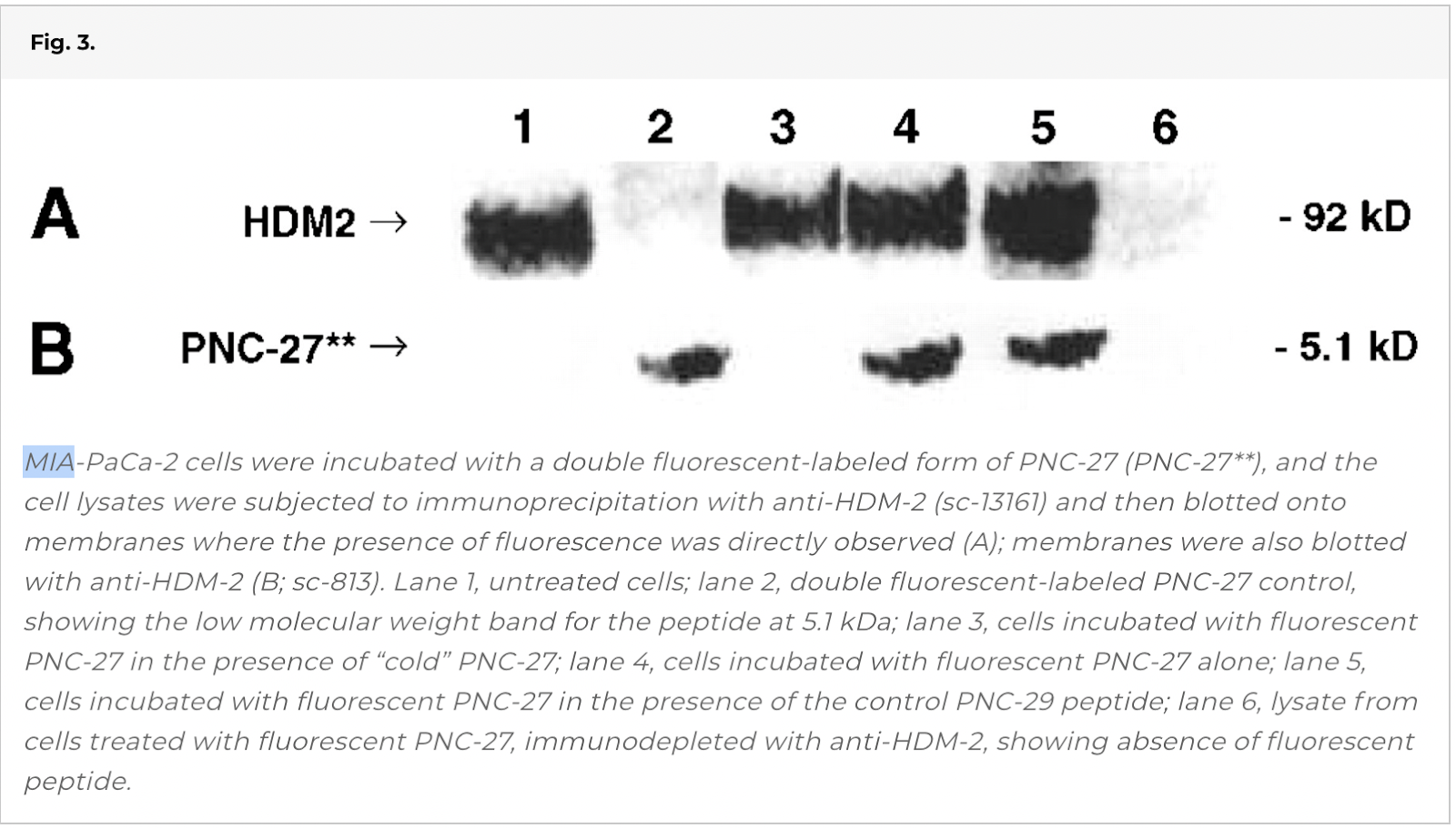
Coimmunoprecipitation of HDM-2 with Fluorescent-Labeled PNC-27.
We next sought to determine whether this peptide binds to HDM-2 in cancer cells. We incubated MIA-PaCa-2 cells with the double fluorescent-labeled form of PNC-27 and immunoprecipitated (IP) HDM-2.
Lane 4 of Fig. 3 shows that IP HDM-2 contains a significant amount of the fluorescent-labeled peptide. In contrast, as shown in lane 3, if the cells are incubated with equimolar concentrations of fluorescent-labeled PNC-27 and unlabeled PNC-27 or closely related PNC-28 (1–3), the 5.1 kDa fluorescent band is no longer present. In contrast, if the cells are incubated with equimolar concentrations of the double-labeled peptide and the negative control peptide PNC-29, the fluorescent band is present as shown in lane 5. These results suggest specific binding of PNC-27 to HDM-2.
Colocalization of PNC-27 with HDM-2 in the Cancer Cell Membrane.
We obtained further evidence for the interaction of PNC-27 with HDM-2 in cancer cell membranes by incubating PNC-27 with two different cancer cell lines, A2058 and MCF-7, and then incubating the cells with FITC- (green-fluorescent-) labeled anti-PNC-27 and TAMRA- (red-fluorescent-) labeled anti-HDM-2. We then viewed the cells using confocal microscopy. Colocalization of PNC-27 with HDM-2 in the cell membrane should result in concurrent green- and red-fluorescent signals from the two antibodies, presenting as yellow fluorescence in the cell membrane.
As shown in Fig. 4 A, A-2058 cells treated with PNC-27 show strong green (PNC-27, DO1 antibody) and red (HDM-2) fluorescence uniquely in the membrane of these cells; the latter result confirms the results of Fig. 2. In the third frame of Fig. 4 A, there is pronounced yellow fluorescence in the membrane, suggesting colocalization. Fig. 4 B shows identical results for MCF-7 cells treated with PNC-27. In control experiments, we found that incubation with DO1 antibody to PNC-27/p53 of both cell lines that were not treated with PNC-27 did not show green fluorescence in the cell membrane, indicating that p53 was not present in this fraction. Treatment of two untransformed cell lines, i.e., BMRPA1 and MCF-10-2A, with PNC-27 followed by incubation with the two labeled antibodies resulted in identical patterns of fluorescence in which green fluorescence was diffusely distributed throughout the cells, suggesting that the peptide entered the cells without being held in the membrane, whereas there was no red fluorescence in their membranes, confirming our findings in Fig. 2 showing the absence of HDM-2 in the membrane fractions of untransformed cells by Western blots.
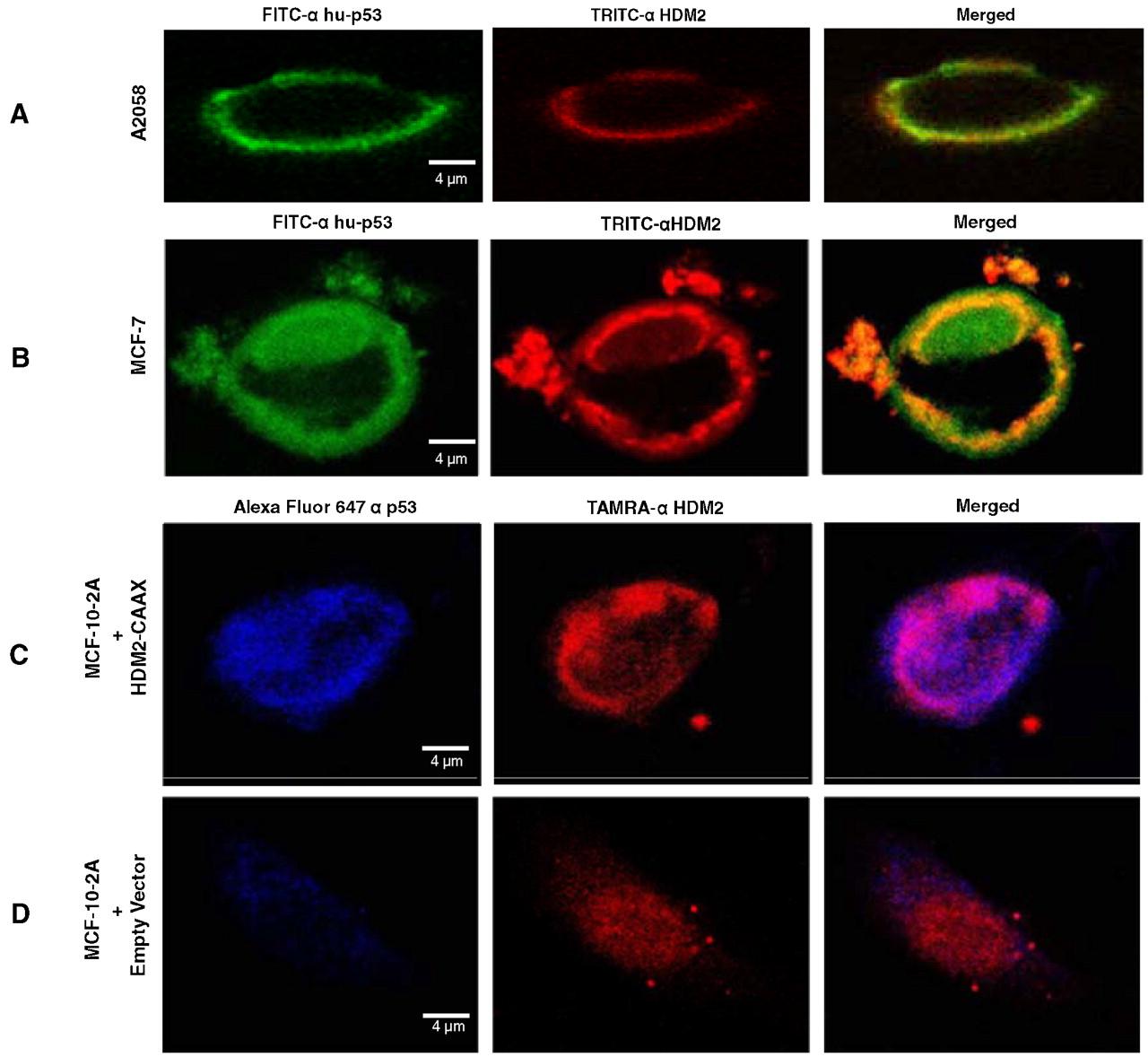
(A and B) Confocal microscopic results from the treatment of A2058 (A) and MCF-7 (B) cells with PNC-27, after which the cells were incubated with green fluorescent anti-PNC-27 DO1 antibody and red-fluorescent anti-HDM-2 antibodies. The fluorophores are labeled for each frame of each panel on the top of the figure. (C and D) MCF-10-2A cells transfected with vectors expressing full-length HDM-2-CVVK membrane-localization peptide (C) and empty vector (D). Blue fluorescence is for PNC-27, red fluorescence is for HDM-2.
Effect of PNC-27 on Untransformed Cells that Are Induced to Express HDM-2 on their Cell Membranes.
We then investigated if expression of HDM-2 in the cell membranes of untransformed cells would make them susceptible to PNC-27. To this end, we transfected MCF-10-2A cells with a vector encoding full-length HDM-2 with a membrane-targeting CAAX (Cys-Val-Val-Lys) sequence (17) and green fluorescent protein (GFP) under a constitutive promoter. For controls, we transfected into MCF-10-2A cells vectors encoding full-length HDM-2 without the presence of CAAX or HDM-2 that lacked residues 1-109 that bind to p53/PNC-27 (15) but contained the carboxyl terminal CAAX sequence (del1-109-HDM-2-CVVK) or empty vector. For each transfected cell line, we found the transfection efficiency to be about 45% as computed from the number of GFP-expressing cells divided by the total number of cells in samples.
We then checked the transfected cells for expression of membrane-bound HDM-2-GFP fusion protein (Mr 122 kDa) by Western blotting of the membrane fractions and whole cell lysates as shown in Fig. 5. Empty vector-transfected cells have no detectable HDM-2 in their membranes (lane 1). Full-length HDM-2-expressing cells contain a small amount of HDM-2 expressed in their membranes that are a small fraction of the total HDM-2 expressed (lane 2). In contrast, both full-length HDM-2-CVVK (lane 4) and del1-109-HDM-2-CVVK (lane 3, Mr 110 kDa that migrates closely to the full-length fusion protein) are expressed at significantly higher levels in the cell membrane. These results confirmed expression of HDM-2 proteins with the membrane-localization sequence in the cell membranes.
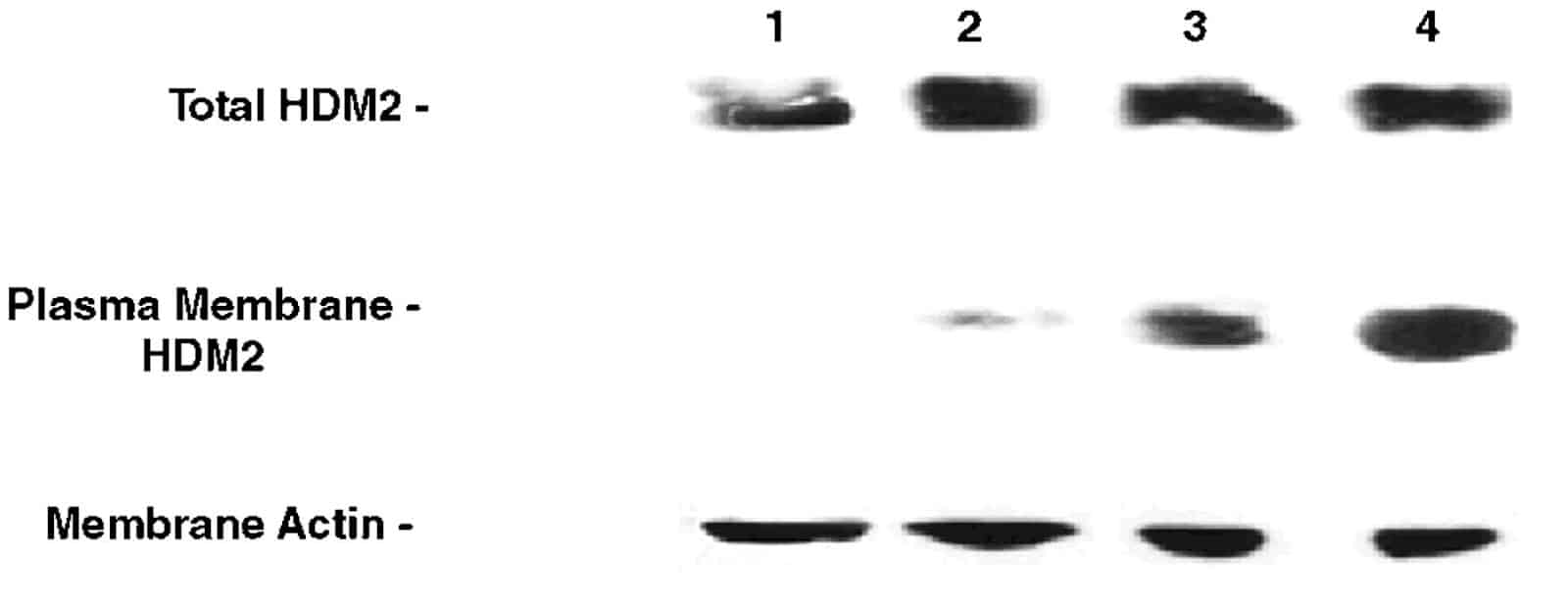
Western blots for expression of HDM-2 in the membrane fractions of each of the four sets of transfected MCF-10-2A cells as follows: lane 1, empty vector-transfected cells; lane 2, cells transfected with plasmid expressing full-length HDM-2; lane 3, cells transfected with plasmid expressing del 1-109 HDM-2-CVVK; lane 4, cells transfected with plasmid expressing full-length HDM-2-CVVK. (Top) Blots for HDM-2 from whole cell lysates. (Middle) Blots for HDM-2 from the membrane fractions. (Bottom) Blots for actin in the membrane fractions.
We then incubated membrane-associated HDM-2-CVVK-expressing cells and HDM-2-expressing cells with PNC-27 to determine if PNC-27 colocalized with HDM-2 in the cell membrane. Fig. 4 C shows that PNC-27 (blue fluorescence) binds to the membrane of HDM-2-CVVK-expressing cells that express high levels of membrane-bound HDM-2 (red fluorescence), confirming the Western blot results in Fig. 5. The last frame of Fig. 4 C shows that there is extensive colocalization of PNC-27 with HDM-2-CVVK in the cell membrane (lavender fluorescence). Fig. 4 Dshows that the PNC-27 signal in the membranes of control cells transfected with empty vector is only minimally present and that the HDM-2 signal is diffuse in the cell and not present in the cell membrane. No colocalization signal is present.
Susceptibility of Transfected Cells to PNC-27.
We further plated each set of transfected cells and treated each with PNC-27 for 24 hr. As shown in Fig. 6, the cells expressing membrane-bound HDM-2-CVVK are induced to release LDH over twice the background value for untreated or empty-vector-transfected cells, and none of the other control cells were found to release LDH above this background value. We also observed a major decrease in cell viability only in the full-length HDM-2-CVVK-expressing cells (Fig. S2A). This decrease was threefold compared with that for untreated cells and almost threefold compared with that for empty-vector-transfected controls. It was also at least 2.5-fold reduced compared with the cell viability of cells expressing HDM-2 on their membranes but lacking the binding site for HDM-2 despite the presence of HDM-2 in the cell membrane fraction (Fig. S2A). None of these sets of cells was induced to undergo apoptosis as revealed by the absence of enhanced caspase activity above background (Fig. S2B), consistent with our prior findings that PNC-27 induces tumor cell necrosis and not apoptosis (1–5). Control peptide PNC-29 (200 μg/ml), had no effect on either LDH release or the viability of any of the four sets of transfected cells, suggesting that PNC-27 binds specifically to HDM-2 on the cell membrane, allowing for its cytotoxicity.
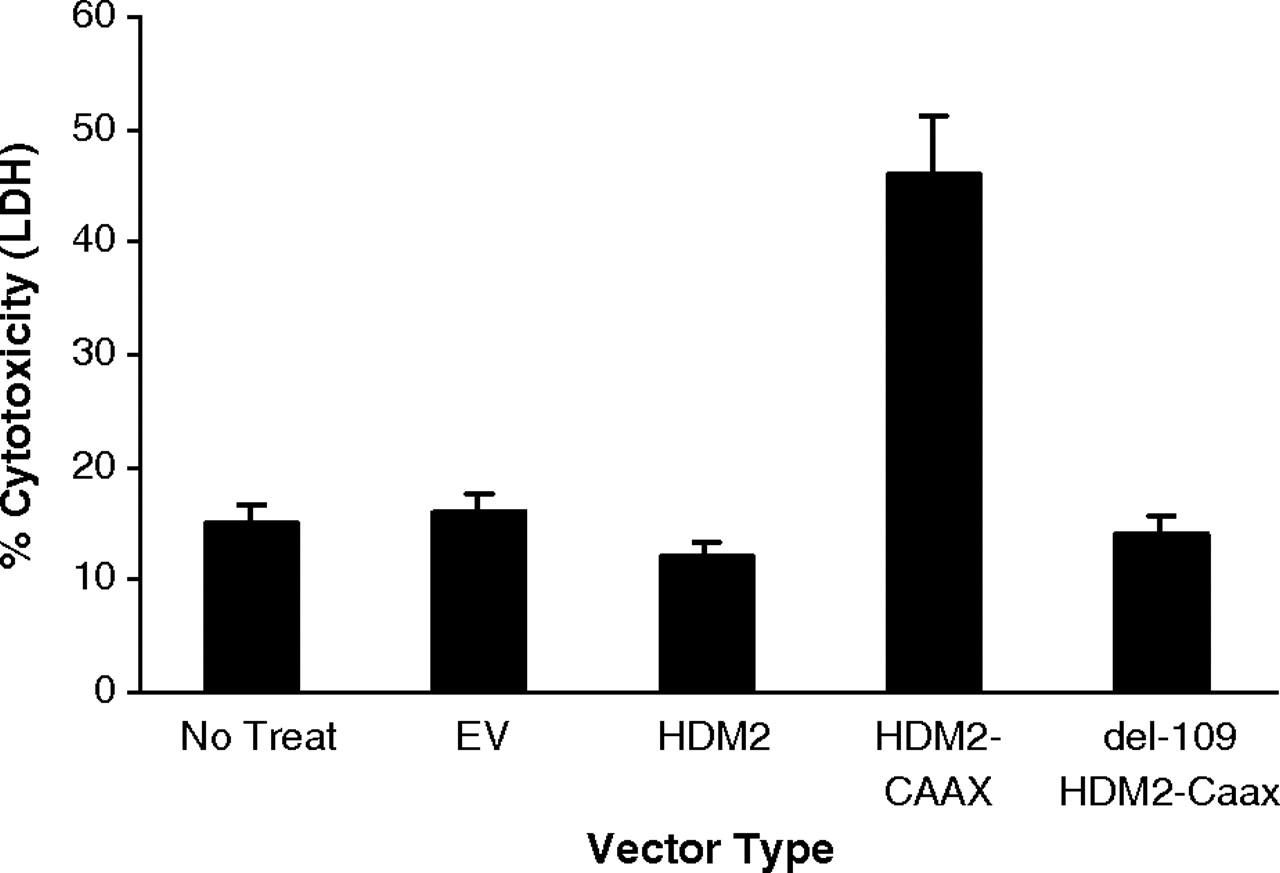
Results of LDH assays for each of the sets of MCF-10-2A cells transfected with Precision Shuttle vectors as labeled on the abscissa for A–C. EV is empty vector; HDM-2 is overexpressed HDM-2; HDM-2 + CAAX is full-length HDM-2 with the membrane-targeting CVVK sequence on the carboxyl terminus; del1-109 HDM-2 + CAAX is HDM-2 without its p53 (PNC-27)-binding domain, residues 1-109, attached on its C terminus to the CVVK membrane-localization sequence.
Discussion
Our present studies suggest that PNC-27 induces cancer cell cytotoxicity by a previously undescribed mechanism in which the peptide induces cancer cell membranolysis that appears to depend on the binding of the peptide to HDM-2 in the cancer cell membrane. HDM-2 is present in the membranes of four different cancer cell lines but is only minimally present in the membranes of three different untransformed cell lines, providing a rationale for the specificity of action of this peptide. These observations are consistent with the results of prior studies on HDM-2 expression in mammalian cells. HDM-2is overexpressed in fast-growing tumors, and the levels of expression of HDM-2 correlated with metastatic potential in primary tumor cell cultures from patients with breast cancer (18). Importantly, HDM-2 was found to colocalize with E-cadherin in the cancer cells’ plasma membranes and to induce its ubiquitination and subsequent degradation, leading to increased cancer cell motility (18). This latter study suggests that HDM-2 is expressed in the membranes of cancer cells and points to a functional role for its presence in the cell membrane.
Binding of PNC-27 to HDM-2 in Cell Membranes Is Critical to its Membranolytic Action.
That PNC-27 interacts with membrane-bound HDM-2 is supported by our confocal microscopy studies on PNC-27-treated cancer cells that show that PNC-27 colocalizes with HDM-2 in the membranes of these cells to which PNC-27 is lethal, a phenomenon that does not occur with untransformed cells that do not express HDM-2 in their membranes and that are not affected by PNC-27.
PNC-27 does not kill MCF-10-2A cells (2). However, transfected cells that express full-length HDM-2-CVVK in their membranes become susceptible to the action of this peptide. In contrast, cells expressing either full-length HDM-2 that does not localize to the cell membrane or HDM-2 that does localize to the cell membrane but does not contain the p53 (PNC-27)-binding domain are much less susceptible to the action of this peptide. These results suggest that the membranolytic action of PNC-27 requires its interaction with HDM-2 in the cancer cell membrane. Similar results to ours have been reported in another study in which plasmid-driven overexpression of MDM-2 in rodent cardiomyocytes rendered these cells susceptible to PNC-28 (19).
Unique Membranolytic Action of PNC-27 May be Due to Its Membrane-Active Conformation.
Using double fluorophore-labeled PNC-27 (as in Fig. 3), we found that the peptide remains intact during tumor cell membranolysis (20). In solution, PNC-27 adopts a membrane-active conformation in which the HDM-2-binding subdomain adopts the x-ray HDM-2-binding conformation. If the membrane-active conformation is retained when the peptide binds to HDM-2 in the cell membrane, then the peptide would remain membrane-active and form pores in the cancer cell membrane, as has been found to occur for other membrane-active peptides (14, 21). When double fluorophore-labeled PNC-27 is incubated with untransformed cells, a small amount of peptide remains in the cell membrane while only the amino terminal domain is found in the nucleus, i.e., the carboxyl terminus is absent, signifying peptide cleavage in the cell (20). This pattern was also found in the colocalization experiments for untransformed cells incubated with PNC-27 (Fig. 4 D). Interestingly, agents, like p53 peptides, that block p53-HDM-2 binding do not induce apoptosis in untransformed cells (4, 6–11). Thus it appears that HDM-2 in the cell membrane blocks the transit of PNC-27 into the cell, allowing it to remain in the membrane where it can undergo pore formation.
This hypothesis can explain why other agents, including small molecules like the nutlins (11), that bind to HDM-2 competitively with p53 in cancer cells, and therefore should also bind to HDM-2 in the cell membrane, induce apoptosis, and not necrosis, of these cells. These agents would not be expected to adopt membrane-active conformations. This includes HDM-2-binding peptides that contain membrane-penetrating sequences on their amino terminal ends (8–10), which we previously found are much less alpha-helical (1) and therefore are not likely to adopt membrane-active conformations.
Materials and Methods
PNC-27, H-Pro-Pro-Leu-Ser-Gln-Glu-Thr-Phe-Ser-Asp-Leu-Trp-Lys-Leu-Leu-Lys-Lys-Trp-Lys-Met-Arg-Arg-Asn-Gln-Phe-Trp-Val-Lys-Val-Gln-Arg-Gly-OH (1 g), was synthesized using solid phase methods (Shaanxi Zhongbang Pharma-Tech Corp., NanguanZhengjie, Xi’an, China) and was > 95% pure by HPLC and mass spectrographic analysis. The bold sequence corresponds to amino acid residues 12-26 of the HDM-2-binding domain of human p53 while the italicized sequence corresponds to the MRP segment that allows entry of the whole peptide into cells. PNC-28, which contains p53 residues 17-26 linked to the MRP, was likewise synthesized by solid phase methods. The negative control peptide, PNC-29 (1–5), containing the X13 peptide from cytochrome P450 (bold) attached to the MRP (italics), H-Met-Pro-Phe-Ser-Thr-Gly-Lys-Arg-Ile-Met-Leu-Gly-Glu- Lys-Lys-Trp-Lys-Met-Arg-Arg-Asn-Gln-Phe-Trp-Val-Lys-Val-Gln-Arg-Gly-OH, was likewise synthesized by solid phase methods and was likewise > 95% pure.
FITC- and TAMRA-double fluorophore-labeled PNC-27 peptide.
This peptide was synthesized at the Biopeptide Corp., La Jolla, CA, with two fluorescent labels: amino terminal 5,6-carboxy-fluorescein (green fluorescence) and carboxyl terminal 5-tetramethyl Rhodamine (TAMRA) (red fluorescence) and was found to be > 95% pure (20).
Plasmids.
Untransformed cells that do not express HDM-2 in their cell membranes are not susceptible to PNC-27 (1–5). To determine if these cells become susceptible to this peptide if they contain HDM-2 in their cell membranes, we have transfected into these cells a plasmid that expresses full-length HDM-2 attached on its carboxyl terminal end to a CAAX localization signal peptide sequence, i.e., Cys-Val-Val-Lys (CVVK), called HDM-2-CVVK. The plasmid was a Precision ShuttleVector with a green fluorescent protein (GFP) tag and a specified open reading frame sequence, in our case, HDM-2-CVVK (Origene, Rockville, MD). Full-length HDM-2 with the carboxyl terminal CAAX sequence, called pHDM2-CVVK, was inserted into this plasmid between Sgf1 and Mlu 1 endonuclease restriction sites. Both the HDM-2 construct and GFP were under a constitutive CMV expression promoter that also induced expression of amp and neo resistance genes.
In addition to this plasmid, we prepared two other plasmids encoding proteins that served as controls: full-length HDM-2 without the CAAX sequence, called pHDM2 and HDM-2-CVVK, that lacks amino acid residues 1-109 that constitute the binding site for p53 and for PNC-27, called pdel1-109-HDM2-CVVK. All plasmids were prepared at Origene. The following primers were employed to construct the DNA sequences encoding each of the HDM-2 proteins. For these sequences all nuclease sites are given in bold; the start (ATG) and stop (TTA) codons are italicized; and the carboxyl terminal codons for the membrane-localization signal sequence, CVVK (CAAX box), are underlined: Full-length HDM-2:
CTACAGCGATCGCCATGGTGAGGAGCAGGCAAATGTGC (+STRAND),
ACGAGACGCGTGGGGAAATAAGTTAGCACAATCATTTG (-STRAND); FULL-LENGTH HDM-2 WITH C-TERMINAL CVVK MEMBRANE-ATTACHING CAAX SEQUENCE
CTACAGCGATCGCCATGGTGAGGAGCAGGCAAATGTGC (+STRAND)
GCGTACGCGTTTACATAATTACACACTTGGGGAAATAAGTTAGCACAATCATTTG G (-STRAND); HDM-2-CVVK WITH RESIDUES 1-109 DELETED
CTACAGCGATCGCCATCTACAGGAACTTGGTAGTAGTC (+STRAND)
GCGTACGCGTTTACATAATTACACACTTGGGGAAATAAGTTAGCACAATCATTTGG (-STRAND)
After digestion with the cloning restriction enzyme Sgf-I and Mlu I, the PCR products were cloned into the Origene Precision Shuttle plasmid pCMV6-AN-GFP with an N-terminal fused GFP-tag. Final constructs were sequenced with VP1.5 5′ GGACTTTCCAAAATGTCG 3′ AND XL39 5′ ATTAGGACAAGGCTGGTGGG 3′ primers.
Cell lines.
The following cell lines were obtained from the American Type Culture Collection (Manassas, VA): MIA-PaCa-(human pancreatic cancer), MCF-7 (human breast cancer), A2058 (human melanoma), A-2058 (human melanoma), MCF-10-2A (normal human breast epithelial cells). Ag13145 cells (primary human fibroblasts, 46 chromosome, XY) were obtained from the Coriell Institute for Medical Research (Camden, NJ) and cultured in DMEM supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Atlanta, GA). In addition, we employed two cell lines that we have developed, i.e., BMRPA1, a normal rat pancreatic acinar cell line, and its k-ras-transformed counterpart pancreatic cancer cell line, called TUC-3 (1), both of which were grown in cRPMI medium as described previously (1).
Methods
Superposition of 2D NMR structures for PNC-27 on that for the p53 17-29 peptide bound to HDM-2.
We superimposed the coordinates for the backbone atoms of the 30 structures that fit the NOE constraints for residues 17-26 of PNC-27 (12) on those for the x-ray structure (15) as described previously (3).
Assays.
Lactate dehydrogenase (LDH) assay using the LDH Cytotoxicity Assay (Promega, Madison, WI); casapse assay for apoptosis [positive control: 2 × 104 cells treated with staurosporine (Sigma, St Louis, MO) (45 μg/ml) (16)]; and MTT cell viability assay were all performed as described previously (2–4). Protein concentrations were performed using the Bradford assay (Pierce, Rockford, IL).
Western blots.
Lysates of 2 × 106cells were either used directly or employed for preparation of purified plasma membranes (22). To assure that the final preparations contained plasma membranes, samples were immunoblotted for membrane β-catenin and by transmission electron microscopy (4). Blotting of both fractions for H(M)DM-2 was performed in a manner similar to that described previously (1–4). Anti-HDM-2 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was used at a dilution of 1∶4,000. Secondary antibody was HRP-conjugated donkey anti-mouse IgG (HRP-anti-M IgG) (Jackson ImmunoResearch, West Grove, PA) used at a dilution of 1∶1,000 in 0.1% milk in TBS-T. Immun-Star HRP Peroxide Buffer + Immun-Star HRP Luminol/Enhancer (ratio 1∶1) (BioRad) was added to the nitrocellulose membranes.
Immunoprecipitation experiments.
To determine if fluorophore-labeled PNC-27 binds to HDM-2 in cancer cells treated with this peptide, we incubated double-fluorophore-labeled PNC-27 (50 μg/ml) (20) with 1 × 106 MIA-PaCa-2 cells for 4 hr. The cells were then lysed (1–4), and 500 μg protein in lysate samples were subjected to IP with 0.5 ug anti-MDM2 mouse monoclonal antibody (D-7, Santa Cruz Biotechnology), 2 μg biotinylated horse antimouse IgG (Pierce) and 30 μl 50% suspension of UltraLink-immobilized NeutrAvidin Biotin-Binding Protein beads. The samples were then electrophoresed in 12.5% PAAG gel (BioRad). The position of the fluorescent peptide was analyzed in the gel with Kodak Image Station 2000R. The proteins were transferred to a PVDF membrane, immunobloted with polyclonal anti-MDM2 antibody (Santa Cruz, N-20), and developed with ECL (Pierce).
Colocalization experiments and confocal microscopy.
These experiments were performed on two different cancer cell lines, A-2058 human melanoma and MCF-7 human breast cancer cells, and on two untransformed cell lines, BMRPA1 rat pancreatic acinar cells (1) and MCF-10-2A untransformed human breast epithelial cells. Cells grown on glass cover slips to 50–60% density were treated for up to 15 min at 37 °C in a humidified 5% CO2—95% air incubator chamber with PNC-27 or PNC-29 (control) at 50 μg/ml incubation medium and were then washed and fixed in 3% paraformaldehyde in PBS (pH 7.2) supplemented with 0.01% glutaraldehyde for 1.5 h followed by extensive washing and transfer into PBS for storage until mounting on glass slides for microscopy. Free aldehyde groups were quenched by incubating cells with glycine (0.2 M) and sodium borohydride (75 mM) followed by washing in PBS. Cells were then stained (direct staining) for 2 h, 4 °C, with fluorescein-labeled mouse monoclonal antibody against p53 [FITC- mAbα-p53 (DO-1)] (5 μg/ml) and rhodamine-labeled (TRITC-) mAbα-against H/R/MDM-2 (5 μg/ml) (both labeled antibodies, Pierce) in 1% FBS-PBS. After removal of nonreactive Ab and extensive washing, the cover slips were mounted on glass slides over Prolong Gold Antifade (Molecular Probes, Invitrogen, Carlsbad, CA) and examined with a laser-equipped Olympus Confocal microscope 1 × 76 (Olympus America Inc, Center Valley, PA). Results were digitally recorded. The colocalization of the two antibodies was confirmed by overlapping green (anti-PNC-27) and red (antiH/R/MDM-2) fluorescent labels that produced yellow fluorescence (combined green and red fluorescence).
Dose response experiments.
LDH assays were performed on different cancer cell lines (n = 3–5) that were incubated for 30 min with PNC-27 over a concentration range of 10 μg.ml/1 mg/ml as described previously (2–4).
Transfection of HDM-2 constructs into untransformed cells.
The plasmids that were constructed as described in Materials above were transfected into untransformed MCF-10-2A cells using the procedures described previously (4). The transfection efficiency was evaluated by analyzing the GFP fluorescence at 480 nm.
Treatment of transfected cells with PNC-27 or PNC-29.
The transfected cells in media were then incubated at 37 °C with 5% CO2 for 24 h, at which time they were treated with PNC-27 or PNC-29 peptide (sonicated briefly prior to addition) such that the final concentration was 300 μg/ml. Samples were assayed for LDH, MTT, and caspase. In addition, samples were processed for confocal microscopy as described above except for the following modification. Because the cells contained GFP, to localize PNC-27, it was necessary to use another fluorescent probe other than green-fluorescent FITC-labeled DO1 antibody. Cells were incubated with unlabeled DO1 and anti-HDM-2 as described above. The cells were then washed and incubated with Alexa Fluor 647 goat antimouse IgG (1∶200) (against DO1 mouse) (Invitrogen–Molecular Probe, Eugene, OR) and TAMRA-labeled goat antirabbit IgG (1∶200) (against anti-HDM-2 rabbit polyclonal IgG) (Sigma, St. Louis, MO). The cells were processed for confocal microscopy, and the membrane fractions and whole cell lysates were blotted for either HDM-2 or actin [rabbit anti-actin-42 polyclonal antibody (1∶5000)] (Sigma).
Targeting Membrane HDM-2 by PNC-27 Induces Necrosis in Leukemia Cells But Not in Normal Hematopoietic Cells [2]
Membrane HDM-2 is selectively expressed at high levels in human leukemia U937, OCI-AML3 and HL60 cells. We examined the expression of HDM-2 on the cell surface of CD34-U937, OCI-AML3 and HL60 using flow cytometry. Human monocytes do not grow well in vitro (unpublished observation and personal communication). Hence, we chose normal rat mononuclear cells as negative control cells and compared the expression of HDM-2 in these cells to leukemia cells. As shown in Figure 1, a large population of leukemia cells expressed membrane HDM-2 and we noticed a considerable shift in median fluorescence of HDM-2 in leukemia cells compared to their isotype control (IgG). In contrast, there was a negligible shift in median fluorescence of HDM-2 in rat mononuclear cells compared to isotype control groups. This indicates significant expression of membrane HDM-2 in the tested human leukemia cells but not in normal rat mononuclear cells (MNCs).
PNC-27 binds to membrane HDM-2 in vitro. To test if membrane HDM-2 can colocalize with PNC-27, cells were treated with PNC-27 for 1 h. Cells were then incubated with rabbit anti-HDM-2 fluorescently labeled with red anti-rabbit antibody and mouse anti-p53 fluorescently labeled with green anti-mouse antibody. If the two signals are localized in the same area, yellow fluorescence is emitted indicating colocalization.
Confocal images of U937, OCI-AML3 and HL60 (Figure 2A, B and C) further confirmed the expression of HDM-2 in the membrane by the significant red signal emitted due to localization of red anti-HDM2 in the membrane. Similarly, significant green fluorescence was observed at the cell surface as a result of PNC-27 localization in the membrane. It should be noted here that U937 and HL60 are p53 null cell lines and hence, the green fluorescence emitted is completely from the interaction between green anti-p53 and PNC-27 (DO-1 anti-p53 binds to PNC-27, a peptide construct derived from p53) on the cell surface. Furthermore, even though OCI-AML3 has wildtype p53, p53 is not stable at the protein level and expresses no significant levels of p53 protein (14). Hence, we can conclude that the green fluorescence in OCI-AML3 cells is a result of PNC-27 localized at the cell membrane. We observed strong binding of HDM-2 and PNC-27 as evidenced by significant yellow fluorescence at the cell surface. This confirms that PNC-27 can bind to membrane HDM-2 of human leukemia cells. Absence of green fluorescence in PNC-29 treated U937, OCI-AML3 and HL60 cells indicated that the green anti-p53 antibody staining is specific to PNC-27.
PNC-27 is selectively cytotoxic to human leukemia cells but not to normal rat mononuclear cells.MTT assay was used to assess the impact of PNC-27/HDM-2 association on cell viability. We noticed a dose dependent decrease in cell viability of U937, OCI-AML3 and HL60 cells after 4 h. PNC-27 induced cell death in U937 with greater efficiency at very low IC50 of 4.7 μM as compared to the other two AML cell lines. The IC50s for OCI-AML3 and HL60 were 83.9 μM and 91.1 μM, respectively. In addition, PNC-27 was tested in normal rat MNCs and we observed no PNC-27-induced cytotoxicity in normal cells. The negative control peptide PNC-29 did not demonstrate any effect on the viability of cancer and normal cells (Figure 3). Therefore, PNC-27 induces its cytotoxic effects selectively in leukemia cells without any toxicity towards normal mononuclear cells even at a dose higher than the highest IC50 observed. This observation is consistent with our previous studies where we observed PNC-27 induced anti-cancer activity in human leukemia K562 cells while sparing the normal murine lymphoid cells (3).
Necrosis is the mechanism of anti-leukemic activity of PNC-27. To investigate the mechanism of cell death, levels of necrotic and apoptotic markers were measured after treatment with different concentrations of PNC-27. After 4 h, there was a dose dependent release of LDH in culture supernatants which indicates necrotic cell death as shown in Figure 4. In addition, the LDH assay confirmed the absence of PNC-27-mediated cytotoxicity in normal rat mononuclear cells. PNC-29 did not increase LDH levels in the media of cancer or normal cells.
We then studied the effect of PNC-27 on early and late apoptotic markers. Apoptosis begins with exposure of phosphatidylserine on the outer plasma membrane and can be detected by staining the cells with annexin V. Staurosporine (STS), a known inducer of apoptosis, was used as a positive control. There was no significant staining of cells with annexin V, when treated with PNC-27 (Figure 5). Since this early event is reversible, we confirmed the findings by testing the effect of PNC-27 on caspase-3 activation which is a later apoptotic event. As shown in Figure 6, PNC-27 had no effect on caspase-3 whereas STS induced significant caspase-3 activity. These observations are consistent with our previous findings in solid and non-solid tumors which suggest that PNC-27 induced cell death by necrosis.
Potential Adverse event [3]
We present a case of massive GI hemorrhage following a patient receiving experimental PNC-27 treatment in Mexico. A 46-year old female with end-stage metastatic cervical cancer who had underwent multiple courses of chemotherapy and a pelvic exenteration surgery presented with hematemesis. She had exhausted her treatment options in the U.S. and sought experimental PNC-27 treatment in Mexico two weeks prior to admission. Two days following PNC-27 infusion, she developed large volume hematemesis along with bloody discharge from her urostomy and colostomy. She came back to the U.S. and presented to a satellite facility where she was started on IV pantoprazole and octreotide infusion and received six units packed red blood cells prior to transfer to our facility. On presentation, she was hypotensive and tachycardic with physical exam remarkable for bright red output from her urostomy and colostomy. Labs revealed hemoglobin 9.4 g/dL, platelets 132 K/mm3, INR 1.4, fibrinogen activity 473 mg/dL along with normal serum chemistries. Upper endoscopy showed large amounts of blood in the stomach and a two centimeter pigmented gastric ulcer that was treated endoscopically with epinephrine and endoclip placement. Shortly after intervention the patient continued to have coffee ground emesis and dark ostomy output requiring frequent transfusions and later perished after transition to comfort care. PNC-27 is a peptide that contains the HDM-2 binding domain of p53 and causes tumor cell death by membranolysis. There is currently no published data on its efficacy or side effect profile in humans. We present a case of massive GI hemorrhage following experimental PNC-27 treatments in Mexico. It is difficult to prove causation in this case, however the temporal relationship between PNC-27 treatment and the patient’s presentation raises concern for potential adverse event. Reporting of possible events related to experimental treatments is crucial to inform the public of the potential associated harms. [3]
- Ehsan Sarafraz-Yazdi, Wilbur B. Bowne, Victor Adler, +8, Kelley A. Sookraj, Vernon Wu, Vadim Shteyler, Hunaiz Patel, WilliamOxbury, Paul Brandt-Rauf, Michael E. Zenilman, Josef Michl[email protected], and Matthew R. Pincus[email protected] -8Authors Info & Affiliations Edited by Harold A. Scheraga, Cornell University, Ithaca, NY, and approved November 17, 2009 (received for review August 28, 2009)
January 11, 2010 107 (5) 1918-1923 https://doi.org/10.1073/pnas.0909364107
- Targeting Membrane HDM-2 by PNC-27 Induces Necrosis in Leukemia Cells But Not in Normal Hematopoietic Cells
ANUSHA THADI, LAUREN LEWIS, EVE GOLDSTEIN, ANSHU AGGARWAL, MARIAN KHALILI, LINDSAYSTEELE, BORIS POLYAK, SHABNAM SEYDAFKAN, MARTIN H. BLUTH, KRISTINE A. WARD, MICHAELSTYLER, PAUL M. CAMPBELL, MATTHEW R. PINCUS, WILBUR B. BOWNEAnticancer Research Sep 2020, 40 (9) 4857-4867; DOI: 10.21873/anticanres.14488
- Aguon, Paul Muna MD1; Aasen, Tyler DO1; Distler, Edward S. DO1; Mallin, Emily MD2 Experimental PNC-27 Therapy and Massive GI Hemorrhage: A Complication or Coincidence?, American Journal of Gastroenterology: October 2017 – Volume 112 – Issue – p S1035-S1036