THE PRODUCTS PURCHASED ON THIS WEBSITE ARE INTENDED FOR RESEARCH CHEMICAL USE ONLY. These products are not intended to be used for human or animal consumption and/or ingestion of any kind. These products should not be used as food additives, drugs or household chemicals. These products should only be used by Qualified Professionals.
Bodily introduction into Human and Animals of any kind is strictly forbidden by law.
All the product information on this website is for educational purpose only.
PTD-DBM and Valproic Acid associated with Hair Regrowth and Wound-Induced Hair Neogenesis
Product Usage: THE PRODUCT IS INTENDED AS A RESEARCH PURPOSE ONLY. This designation allows the use of research chemical strictly for in vitro testing and laboratory experimentation only. All product information available on www.peptidesworld.com is for educational purposes only. Bodily introduction of any food, cosmetic and may not be misbranded, misused or mislabeled as a drug, food or cosmetics.
Targeting of CXXC5 by a Competing Peptide Stimulates Hair Regrowth and Wound-Induced Hair Neogenesis
Highlights:
- Inhibition of CXXC5 Disheveled interations maintains dermal papilla cells
- Cotreatment with valproic acid synergistically augments hair follicle regeneration in CCXC5 deficient mice.
- Targeting CXXC5-Disheveled interations can be a strategy for therapy of hair loss patients, such as with interfering short peptides.
WNT/β-catenin signaling is a tightly regulated pathway, with roles in carcinogenesis, embryonic development, and tissue regeneration throughout development and adult life. For example, WNT of hair follicle morphogenesis and hair follicle cycling (Myung and Ito, 2012). Not surprisingly ,aberrant regulation of WNT signaling is associated with hair loss or alopecia plays a crucial role in regulation.
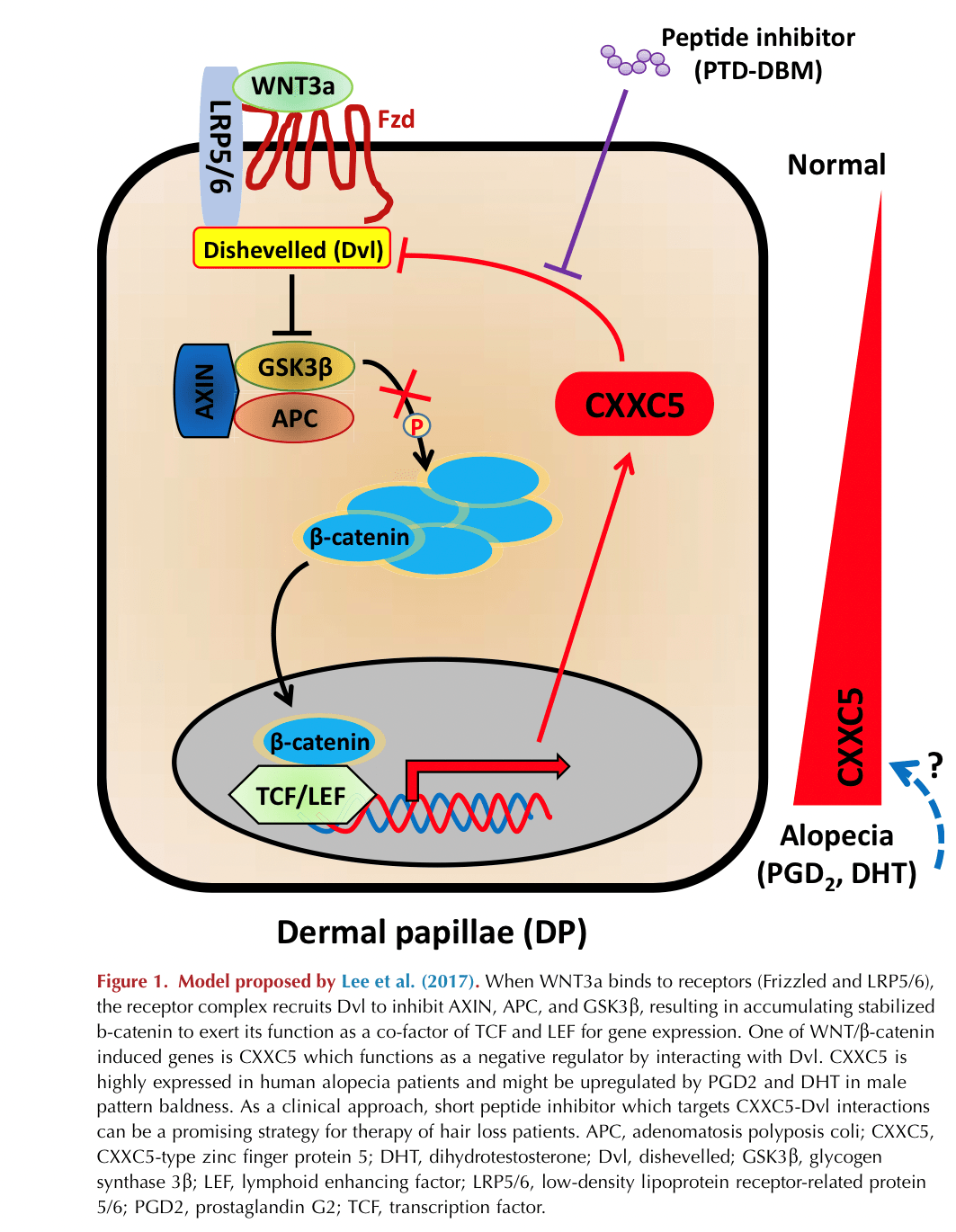
Binding of WNT to its cell surface receptor Frizzled (Fzd) triggers intracellular signaling cascades by recruiting Disheveled (Dvl) to the receptor complex consisting of adenomatosis polyposis coli, axin, and Glycogen synthase kinase 3 beta (GSK3β) which in turn protects β-catenin from proteasomal degradation. As a result, β-catenin is no longer phosphorylated and it accumulates in the cytoplasm, available for translocation into the nucleus where it functions as a cofactor for other transcriptional factors (lymphoid enhancer-binding factor/transcription factor) to induce expression of WNT transcriptional targets. Activation of WNT signaling can be repressed by disparate extracellular inhibiting proteins with different modes of functions (
Lim and Nusse, 2013). First, dickkopfs are secreted cysteine-rich proteins that bind to low-density lipoprotein receptor-related proteins (LRP5/LRP6; WNT coreceptors) and inhibit WNT signaling. Secondly, secreted Fzd-related proteins also contain cysteine-rich domains and block WNT signaling by binding to WNTs and Fzd receptors. Lastly, WNT inhibitory factors are secreted lipid-binding proteins that bind to WNT ligands directly, preventing their binding to Fzd receptors. Numerous studies have demonstrated the importance of WNT signaling in hair follicle regeneration by altering the expression of these inhibiting molecules. However, the precise regulatory mechanisms of WNT remain incompletely characterized due to myriad different types of WNTs, Fzd receptors, and inhibitors.
CXXC5, mostly localizing to the cytosol and nucleus, is a member of CXXC-type zinc finger proteins that interacts with Dvl and functions as a negative regulator of WNT signaling. Previous studies demonstrated that CXXC5 has multiple important functions. In particular,
Lee et al., 2015 have previously reported that CXXC5, as a negative regulator of WNT signaling, can modulate wound healing. Despite the demonstrated importance of WNT/β-catenin signaling in hair regeneration, the inhibitory capacity of CXXC5 for this pathway has not yet been studied in this context. Thus, as an extension of their prior work,
Lee et al., 2017 investigate how CXX5-Dvl interactions impact hair regeneration. The authors suggest that the inhibition of CXXC5-Dvl interactions can be a therapeutic strategy to treat hair loss patients.
The authors first examined the expression pattern of CXXC5 in comparison with β-catenin in hair follicles of haired and bald scalp tissues from patients with androgenetic alopecia according to the hair cycle. They found that expression of CXXC5 and β-catenin were inversely correlated to each other in both haired and bald scalp, suggesting that the level of CXXC5 is associated with hair loss. Interestingly, CXXC5 is expressed in hair follicle dermal papilla (DP) cells, although it is also expressed in hair follicle keratinocytes and arrector pili muscles. They further investigated the role of CXXC5 in DP cells by measuring alkaline phosphatase (a hallmark marker of DP absent in normal fibroblasts) and proliferation activity. Of note, DP cells have been known to lose their original characteristics including morphological identity and proliferative activity after a few in vitro passages (
Driskell et al., 2011). Here, the authors show that CXXC5 inhibits alkaline phosphatase activity and proliferation via interaction with Dvl-1 using overexpression vectors and small interfering RNA in DP cells, suggesting that CXXC5 may inhibit DP cell identity. These results raise questions such as how DP cell identity is regulated by the level of CXXC5 during culturing, given the normal positive WNT requirement (
Kishimoto et al., 2000). Also, could CXXC5 indirectly or directly influence other known players, such as Shh or FGF, in DP cell formation? Does CXXC5 assist in fibroblast fate decisions?
In the next set of experiments, the authors evaluated hair regrowth in CXXC5-deficient mice and found that loss of CXXC5 supported the transition from telogen to anagen with increased levels of β-catenin, alkaline phosphatase, and proliferating cell nuclear antigen, again consistent with a WNT-inhibitory function of CXXC5. Moreover, the treatment of CXXC5 null mice with valproic acid (VPA)—a GSK3β antagonist with some wnt agonist activities—showed synergistic effects on hair regrowth compared with untreated wild-type mice. For further evaluation, the authors used a specific peptide labeled protein transduction domain-Dvl-binding motif (a competitive inhibitor) that inhibits the binding of CXXC5 to Dvl and found that the cotreatment of protein transduction domain-Dvl-binding motif with Wnt3a or VPA synergistically stimulates DP cell activities and hair regrowth.
Interestingly, Wnt3a induces CXXC5 expression, which subsequently disrupts β-catenin stability, suggesting that CXXC5 functions as a negative feedback regulator of WNT/β-catenin signaling. Finally, the authors examined the interaction of CXXC5 with Dvl in the context of wound-induced hair neogenesis. As seen in normal telogen mice, blocking CXXC5-Dvl interactions promoted hair follicle regeneration and cotreatment of VPA synergistically enhanced wound-induced hair neogenesis activity with significantly augmented expression of Fgf9 and keratin 17 as well as β-catenin, alkaline phosphatase, and proliferating cell nuclear antigen. These results support multiple overlapping roles for CXXC5.
This study brings up several interesting ideas for further studies. First, other than Wnt3a, what factors induce CXXC5 expression in a bald scalp? Because it has been determined that levels of prostaglandin D2 (
Garza et al., 2012) and dihydrotestosterone are high in bald scalps and that they inhibit hair follicle regeneration, it might be interesting to determine if prostaglandin D2 and dihydrotestosterone stimulate CXXC5 expression in male pattern baldness (Figure 1). Second, recent studies reported that CXXC5 can bind the histone methyltransferase SUV391H1, and its ortholog induces Tet2 expression (
Tsuchiya et al., 2016), suggesting that CXXC5 could have a role in epigenetic regulation, perhaps of WNT signaling. Lastly, the authors show that blocking CXXC5-Dvl interactions can increase new hair follicle generation in the wound-induced hair neogenesis system. Because synthetic peptides can be tested in humans, can protein transduction domain-Dvl-binding motif be injected into the human scalp with deficient WNT signaling?
Soung-Hoon Lee, Seol Hwa Seo, Dong-Hwan Lee, Long-Quan Pi, Won-Soo Lee, Kang-Yell Choi
Targeting of CXXC5 by a CompetingPeptide Stimulates Hair Regrowth andWound-Induced Hair Neogenesis
Journal of Investigative Dermatology Volume 137, Issue 11, Pages 2248-2250