CJC 1295 DAC 2mg
$49.00
CJC-1295 DAC is a synthetic human GHRH analog that selectively and covalently binds to endogenous albumin after subcutaneous administration, thereby extending its half-life and duration of action. Studies in animals (rats, pigs, and dogs) have shown that several days after a single administration of CJC-1295, serum IGF-I levels are still increased.
- Buy 5 and save 10%
- Buy 10 and save 15%
Legal Notice Regarding This Product
THIS PRODUCT IS STRICTLY INTENDED FOR RESEARCH USE ONLY. It is designated solely as a research chemical. Usage is confined to in vitro testing and laboratory experimentation exclusively. Information available on our website about this product is for educational purposes only and should not be interpreted as an endorsement or directive for use outside these bounds. Introducing this product into humans or animals by any route is strictly prohibited by law. Handling of this product should be undertaken only by licensed, qualified professionals.
This product is not to be used as a drug, food, or cosmetic. Misbranding, misusing, or mislabeling this product as a drug, food, or cosmetic is illegal. Users are responsible for complying with all applicable laws and regulations regarding the handling and use of this product.
THE PRODUCTS PURCHASED ON THIS WEBSITE ARE INTENDED FOR RESEARCH CHEMICAL USE ONLY. These products are not intended to be used for human or animal consumption and/or ingestion of any kind. These products should not be used as food additives, drugs or household chemicals. These products should only be used by Qualified Professionals.
Bodily introduction into Human and Animals of any kind is strictly forbidden by law.
All the product information on this website is for educational purpose only.
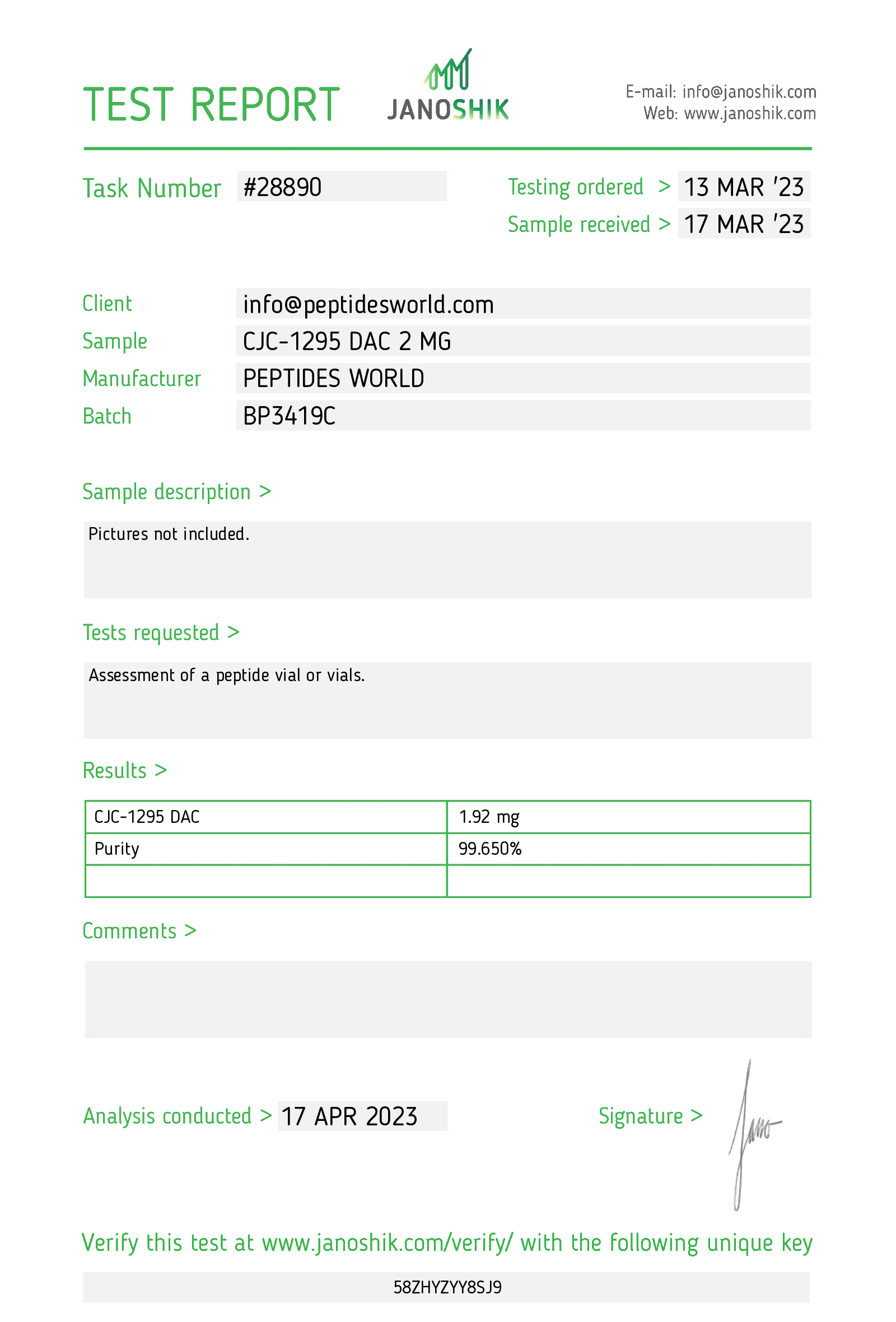
CJC-1295, a long-acting growth hormone-releasing hormone (GHRH) analog
HIGHLIGHTS (observed in animal studies):
-
- Increase of IGF-1
CJC-1295 DAC is a synthetic human GHRH analog that selectively and covalently binds to endogenous albumin after subcutaneous administration, thereby extending its half-life and duration of action. Studies in animals (rats, pigs, and dogs) have shown that several days after a single administration of CJC-1295, serum IGF-I levels are still increased.
CJC-1295 is a synthetic human GHRH analog that selectively and covalently binds to endogenous albumin after subcutaneous administration, thereby extending its half-life and duration of action. Studies in animals (rats, pigs, and dogs) have shown that several days after a single administration of CJC-1295, serum IGF-I levels are still increased (5). In a recent report, CJC-1295 administered to healthy subjects has been proven to be effective in causing a sustained dose-dependent stimulation of serum GH and IGF-I levels when injected at intervals of 1–2 wk (14).
The primary aim of this study was to determine the minimum frequency of injections that could correct the GHD phenotype in the GH-deficient GHRHKO mice, as measured by improvement of auxological parameters and body composition. Second, we wanted to assess the stimulatory effect of CJC-1295 on somatotroph cell proliferation. We show that once-daily injections of 2 μg of CJC-1295 are able to completely normalize growth. Furthermore, administration of the same dose of CJC-1295 every 48 or 72 h produced intermediate results, indicating an interval-dependent effect.
Structure:
Formula: C152H252N44O42
Molecular Mass: 3367.954 g·mol−1
CAS: 446262-90-4
Material and Method
Animals.
Male offspring derived from knockout breeding pairs on a hybrid C57BL/6-SV129 background were used for all GHRHKO groups, while male heterozygous animals (HTZ; also on a C57BL/6-SV129 background) served as a control group for normal growth and GH status. HTZ rather than wild-type C57Bl/6 or SV129 mice were used to ensure a closer match between the genetic background of GHRHKO mice and controls. Mice were weaned during the treatment onto a standard mouse/rat chow (Prolab RMH2500; PMI Nutrition International, Brentwood, MO) at 4 wk of age and were housed in groups of four animals based on genotype and treatment. All animals experienced a controlled environment with 14:10-h light-dark cycles at 21°C and 23% humidity. Food and tap water were supplied ad libitum. All procedures were approved by the Johns Hopkins Institutional Animal Care Committee.
Treatment with CJC-1295.
Three groups of 1-wk-old GHRHKO mice were treated for 5 wk with 2 μg of CJC-1295 at intervals of 24 h (CJC/24 h; n = 8), 48 h (CJC/48 h; n = 8), and 72 h (CJC/72 h; n = 7). Two placebo (pbo)-treated groups (treated daily) were included to allow comparison with the active treated animals: GHRHKO mice (GHRHKO/pbo; n = 8) and mice HTZ for the GHRHKO allele that have normal growth parameters (HTZ/pbo). Drug or placebo was injected in the morning between 0800 and 0900 (24-h clock). At the end of the treatment period, CJC/24 h, CJC/48 h, and CJC/72 h mice were killed 24, 48, and 72 h after their last dose, respectively, to determine the time-dependent effect of CJC-1295 on GH and IGF-I production. Because of the delicate abdominal wall structure in 1-wk-old mice, the drug was injected subcutaneously in the interscapular area during the first week of treatment and in the abdominal area starting the second week of treatment. Mice within the same litter were assigned to different groups to avoid any influence of litter size and maternal breast-feeding behavior.
Auxological parameters.
Body length, naso-anal length (N-A), and total body weight (TBW) were measured at baseline and weekly thereafter, using a daily calibrated electronic balance (Scout Pro Balance; Ohaus, Pine Brook, NJ) and an electronic digital caliper (Control, Friendswood, TX). At the end of the treatment period, measurements of femur and tibia length were obtained by dissection of the surrounding muscle tissues and disarticulation without disturbing the articular cartilages, using an electronic digital calliper. Femur length was measured as the maximal distance between the head of the great trochanter and the distal condyles, while the tibia length was considered as the maximal distance between the proximal condyles and malleolus.
To determine the effects of treatments on body composition, weights of subcutaneous fat pad (SF), visceral fat (VF), and lean mass (LM) were assessed separately using a precision electronic balance (AR1140; Ohaus). After removal of skin, the perirenal and epidydimal fat pads were pooled for VF measurement, and the sum of the fat pads from the interscapular and axillary region, thighs, and inguinal region was used for SF measurement.
LM was determined by weighing animals deprived of tail, skin, adipose tissue, and organs. LM, VF, and SF weights of each animal were normalized to TBW by calculating the percentage as follows: [weight (g)/TBW (g)] × 100.
Pituitary total RNA and GH and prolactin mRNA.
Because of the extremely small pituitary size (∼1 mm), the weight of the gland could not be measured directly. Similarly, three glands from each group were pooled for RNA extraction. Quantification of pituitary mass was extrapolated by measuring the mean total RNA obtained from a pool of three pituitary glands of each group. Total RNA was isolated using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer’s recommendations. The average total RNA yield for each group was quantified spectrophotometrically at 260/280 nm (DU 640 Spectrophotometer; Beckman-Coulter, Fullerton, CA).
Pituitary GH and prolactin mRNA contents were measured by Northern analysis as previously described using 3 μg of total RNA (1) for each group. Results were normalized to mouse GAPDH mRNA expression after stripping of the membrane and quantified by phosphor imager (Molecular Imager FX; Bio-Rad, Life Science Group, Hercules, CA) as previously described (1).
Pituitary GH protein.
Pooled pituitary extracts used for RNA isolation were also used for protein extraction according to TRIzol manufacturer recommendations. Equal amounts of protein from each group were resolved on 15% SDS-PAGE and electrotransferred onto polyvinylidene difluoride membrane (Immobilon-P; Millipore, Bedford, MA). After overnight blocking in Tris-buffered saline, 0.02% Tween 20, and 5% milk at 4°C, the membrane was incubated for 2 h at room temperature with rabbit anti-mouse GH antibody (National Hormone and Peptide Program; Harbor UCLA Medical Center, Torrance, CA) at a 1:80,000 dilution. After a washing, the membrane was incubated for 1 h with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (1:3,000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA). Band size was determined by comparison with a full-range protein weight marker (Precision plus protein; Bio-Rad).
Pituitary immunohistochemistry.
After removal of skin from the heads, parietal and temporal bones were removed, and tissues were fixed in 10% buffered Formalin for 24 h and decalcified (Cal-Ex; Fisher Scientific, Fair Lawn, NJ) for 1 wk. After bisection of the brain through the dorsal sagittal sinus, the hemispheres were embedded in paraffin and cut into 5-μm sections. The sections were then immunostained for GH and prolactin with rabbit anti-mouse GH and prolactin antibodies (National Hormone and Peptide Program; Harbor UCLA Medical Center) (1:3,000 dilution) using the avidin-biotin-peroxidase complex technique with 3,3′-diaminobenzidine (DAB) substrate (DAKO, Carpinteria, CA) (brown). Cell nuclei were counterstained using hematoxylin (blue).
Serum IGF-I.
On the day of death, blood was obtained by eye bleeding. After centrifugation, sera from all groups were stored at −20°C and assayed together. Serum IGF-I was measured using a rat-mouse IGF-I RIA (DSL-2900; DSL, Webster, TX) after acid ethanol extraction, following the manufacturer’s recommendations. All samples were analyzed in duplicate.
Statistical analysis.
Results are expressed as means ± SE. Parameters were statistically analyzed by ANOVA using an SPSS statistical package, with post hoc analysis by Bonferroni method. Results were considered statistically significant at P < 0.05.
RESULTS
Auxological data and body composition.
CJC-1295 was well tolerated in all animals with no evidence of either local or systemic toxicity. Animals receiving a daily dose of CJC-1295 maintained normal TBW (Fig. 1) and N-A measurements (Fig. 2) through the end of the 5-wk treatment period. Animals treated every 48 and 72 h had significantly greater TBW and N-A compared with GHRHKO/pbo animals, but these measurements remained significantly smaller compared with both HTZ and CJC/24 h mice. Final femur and tibia lengths in both CJC/24 h and CJC/48 h groups were comparable with those of HTZ/pbo but were reduced in CJC/72 h despite being greater than in GHRHKO/pbo (Table 1).
References:
- Alba M, Fintini D, Sagazio A, Lawrence B, Castaigne JP, Frohman LA, Salvatori R. Once-daily administration of CJC-1295, a long-acting growth hormone-releasing hormone (GHRH) analog, normalizes growth in the GHRH knockout mouse. Am J Physiol Endocrinol Metab. 2006 Dec;291(6):E1290-4. doi: 1152/ajpendo.00201.2006. Epub 2006 Jul 5. PMID: 16822960.
CJC 1295 DAC 2mg
$49.00