Legal Notice Regarding This Product
THIS PRODUCT IS STRICTLY INTENDED FOR RESEARCH USE ONLY. It is designated solely as a research chemical. Usage is confined to in vitro testing and laboratory experimentation exclusively. Information available on our website about this product is for educational purposes only and should not be interpreted as an endorsement or directive for use outside these bounds. Introducing this product into humans or animals by any route is strictly prohibited by law. Handling of this product should be undertaken only by licensed, qualified professionals.
This product is not to be used as a drug, food, or cosmetic. Misbranding, misusing, or mislabeling this product as a drug, food, or cosmetic is illegal. Users are responsible for complying with all applicable laws and regulations regarding the handling and use of this product.
THE PRODUCTS PURCHASED ON THIS WEBSITE ARE INTENDED FOR RESEARCH CHEMICAL USE ONLY. These products are not intended to be used for human or animal consumption and/or ingestion of any kind. These products should not be used as food additives, drugs or household chemicals. These products should only be used by Qualified Professionals.
Bodily introduction into Human and Animals of any kind is strictly forbidden by law.
All the product information on this website is for educational purpose only.
HIGHLIGHTS:
⦁ Stable BPC-157, a novel anti-ulcer peptide (1)
⦁ BPC-157: Healing in different tissues
⦁ Tendon Healing (4)
⦁ Bone healing
⦁ BPC-157 & Neuroprotective effect
⦁ Effect on Spinal Cord Injury
⦁ Blood Pressure
⦁ Tendon Healing (4)
⦁ Bone healing
⦁ Stable BPC-157 as anti-ulcer agent (1)
Stable gastric pentadecapeptide BPC 157 is a novel anti-ulcer peptide, used in trials for the treatment of ulcerative colitis and now multiple sclerosis, lethal dose (dosage required to kill 1% of the test population, LD1) not reported.
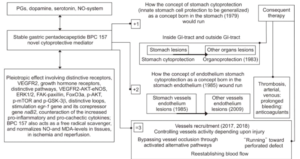
BPC 157 has beneficial effects and thus leads to stomach cytoprotection → organoprotection of the whole gastrointestinal tract, including both prophylactic and therapeutic effects for pre-existing lesions in individuals with the most complex disturbances, such as internal and external fistulas, or anastomosis complicated with severe colitis (indicated as +1). In addition, there is a particular effect on endothelial integrity (indicated as +2). Together, these may result in the particular activation of blood vessels during injury, vessel occlusion, or organ perforation, the recruitment of the vessel to organize an adequate shunting and bypass occlusion (indicated as=3). Furthermore, the effect of BPC 157 is due to its interaction with and modulation of the NO system, and its interaction with prostaglandin, dopamine, and serotonin systems has also been documented. BPC 157 also acts as a free radical scavenger, counteracts free radical-induced lesions, and normalizes NO and MDA levels in tissues and during ischemia and reperfusion. Subsequent studies from other groups have confirmed our original findings.65 Pleiotropic effects involving distinctive receptors, including VEGFR2 and growth hormone receptors, distinctive pathways, including VEGFR2-AKT-eNOS, ERK ½, FAK-paxillin, FoxO3a, p-AKT, p-mTOR and p-GSK-3β, and distinctive loops, including stimulation of the egr-1 gene and its corepressor gene naB2, and counteraction of increases in pro-inflammatory and procachectic cytokines, likely minimize the inherent lack of full understanding of the mechanisms that may be involved. However, more important is the practical evidence from a considerable number of the studies, particularly in gastrointestinal research, that intragastric administration or per os administration in drinking water, is equally effective as injections of the supplement administered to rodents, which has been performed in the majority of studies on BPC 157. In reality, in particular along with its safety profile, LD-1 is not achieved, and there are no reported adverse effects in clinical trials; this evidence suggests the ease of practical clinical application. PGs, prostaglandins; NO, nitric oxide; VEGF, vascular endothelial growth factor; VEGFR2, VEGF receptor 2; eNOS, endothelial nitric oxide synthase; FAK, focal adhesion kinase; FoxO3a, transcription factor; p-AKT, hosphor-AKT; p-mTOR, hosphor mammalian target of rapamycin; p-GSK-3β, hosphor glycogen synthase kinase 3β; MDA, malondialdehyde; GI, gastrointestinal. (1)
⦁ Healing in different tissues
In addition, the other point considers the stomach cytoprotection concept → wound healing concept.1Note, the original cytoprotective agenda (with the generalized cell protection, within the same “cytoprotective” background) includes the improved healing in different tissues.
Indicatively, BPC 157 improves the healing of the skin wounds, muscle, tendon, ligament, and bone injuries. Furthermore, the observed considerable recovery of the skin wound and muscle, tendon, ligament and bone, after severe injury that could be not spontaneously healed may be quite indicative. These healing processes may suggest that BPC 157 may distinctively affect tissue healing (i.e., with BPC 157, tendon heals with the tendon, not with the bone; detached rat Achilles tendon from calcaneus would be properly reattached to calcaneus without surgical intervention). Thus, we suggested that BPC 157 exerts its effect simultaneously in the healing of different tissues, and accommodate the healing processes in different tissues. As a supportive analogy a clear demonstration of the rat fistulas healing appears, and thereby simultaneously achieves healing in different tissues. In rat fistulas studies, creation of the fistulas by anastomoses between the different tissues, accurately provides the defined defect in each of the tissues. Due to the comparative small size of the rats and large size of the defects, these defects would fairly mimic large fistulas in patients that would hardly heal spontaneously. A similar demonstration should be the creation of the different gastrointestinal anastomoses that would accordingly heal as opposed to commonly poor healing in the corresponding control rats. Thus, the healing of the esophagocutaneous, gastrocutaneous, duodenocutaneous, colocutaneous, vesicovaginalis, and rectovaginalis fistulas, assessed grossly, biomechanically and microscopically means demonstration of the both external and internal fistulas healing and consequently, the realization of the simultaneous healing of different tissues. Likewise, is the demonstration of the improved anastomoses healing, gastrointestinal (i.e., esophagogastric, esophagojejunal ileoileal, jejunoileal, colon anastomoses), but also nerve (sciatic nerve) and vascular (abdominal aorta). Consequently, the evidence that it may close and heal the various fistulas and improve anastomosis healing, may be a proof of the fulfilled wound healing concept. (1)
⦁ BPC-157, effect on Tendon Healing (4)
The outgrowth of tendon fibroblasts from tendon explants cultured with or without BPC 157 was examined. Results showed that BPC 157 significantly accelerated the outgrowth of tendon explants. Cell proliferation of cultured tendon fibroblasts derived from rat Achilles tendon was not directly affected by BPC 157 as evaluated by MTT assay. However, the survival of BPC 157-treated cells was significantly increased under the H(2)O(2) stress. BPC 157 markedly increased the in vitro migration of tendon fibroblasts in a dose-dependent manner as revealed by transwell filter migration assay. BPC 157 also dose dependently accelerated the spreading of tendon fibroblasts on culture dishes. The F-actin formation as detected by FITC-phalloidin staining was induced in BPC 157-treated fibroblasts. The protein expression and activation of FAK and paxillin were determined by Western blot analysis, and the phosphorylation levels of both FAK and paxillin were dose dependently increased by BPC 157 while the total amounts of protein was unaltered. In conclusion, BPC 157 promotes the ex vivo outgrowth of tendon fibroblasts from tendon explants, cell survival under stress, and the in vitro migration of tendon fibroblasts, which is likely mediated by the activation of the FAK-paxillin pathway. (4)
⦁ Bone Healing
BPC-157 has been shown to speed up bone healing. A novel stomach pentadecapeptide, BPC-157, improves wound and fracture healing in rats in addition to having an angiogenic effect.
The effect of pentadecapeptide BPC-157 was shown to correspond to improvement after local application of bone marrow or autologous cortical graft. Moreover, a comparison of the number of animals with unhealed defects (all controls) or healed defects (complete bony continuity across the defect site) showed that besides pentadecapeptide intramuscular application for 14 days (i.e., local application of bone marrow or autologous cortical graft), also following other pentadecapeptide BPC-157 regimens (local application, or intermittent intramuscular administration), the number of animals with healed defect was increased. Hopefully, in the light of the suggested stomach significance for bone homeostasis, the possible relevance of this pentadecapeptide BPC-157 effect (local or intramuscular effectiveness, lack of unwanted effects) could be a basis for methods of choice in the future management of healing impairment in humans, and requires further investigation.
⦁ BPC 157 neuroprotective effects
In addition, BPC 157 has neuroprotective effects. It protects somatosensory neurons in capsaicin-treated rats. Sciatic nerve regeneration appears after transection with BPC 157 application, local (into the tube), intragastric or intraperitoneal. After traumatic brain injury, it counteracts the otherwise progressing course. In rat spinal cord compression with tail paralysis, axonal and neuronal necrosis, demyelination, cyst formation, BPC 157 result in marked recovery and rescues tail function in both short-terms and long-terms. After NSAIDs or insulin overdose or cuprizone (a neurotoxin that induces multiple sclerosis like lesion in rat model) or large bowel resection, encephalopathies were attenuated along with gastrointestinal, liver and vascular injuries. When convulsions appeared with paracetamol or insulin, they were completely eliminated or markedly attenuated, much like muscle weakness in cuprizone-rats. (4)
⦁ BPC 157 can improve the healing course of spinal cord injury and lead to functional recovery in rats ⦁ (4)
BPC 157 therapy was administered by a one-time intraperitoneal injection (BPC 157 (200 or 2 μg/kg) or 0.9% NaCl (5 ml/kg)) 10 min after injury. The injury procedure involved laminectomy (level L2-L3) and a 60-s compression (neurosurgical piston (60–66 g) of the exposed dural sac of the sacrocaudal spinal cord). Assessments were performed at 1, 4, 7, 15, 30, 90, 180, and 360 days after injury.
All of the injured rats that received BPC 157 exhibited consistent clinical improvement, increasingly better motor function of the tail, no autotomy, and resolved spasticity by day 15. BPC 157 application largely counteracted changes at the microscopic level, including the formation of vacuoles and the loss of axons in the white matter, the formation of edema and the loss of motoneurons in the gray matter, and a decreased number of large myelinated axons in the rat caudal nerve from day 7. EMG recordings showed a markedly lower motor unit potential in the tail muscle. (4)
⦁ Blood Pressure (3)
In blood pressure studies, compared with l-arginine, pentadecapeptide BPC 157 (without effect on basal normal values) had both a mimicking effect (impaired l-NAME-blood pressure increase, when applied prophylactically and decreased already raised l-NAME values, given at the time of the maximal l-NAME-blood pressure increase (i.e., 10 min after l-NAME)) and preventive activity (l-arginine-induced moderate blood pressure decrease was prevented by BPC 157 pretreatment). When BPC 157 was given 10 min after l-NAME+l-arginine combination, which still led to a blood pressure increase, its previously clear effect (noted in l-NAME treated rats) disappeared. (3)
⦁ BPC 157 may have a particular additional effect during the injury on the blood vessel activity: vessel recruitment to circumvent the vessel occlusion
The final argumentation goes to the findings that BPC 157 may have a particular additional effect during the injury on the blood vessel activity, much like vessel recruitment to circumvent the vessel occlusion, presenting an additional shunting and rapid bypassing loops to rapidly reestablish blood flow integrity. The particular illustration to damage counteraction appears with the ischemic/reperfusion colitis, and rapid bypassing loop through arcade vessels. Consequently, circumventing blockades, blood flow was restored, and pale areas without mucosal folds did not occur. Similar evidence follows the ligation of the superior anterior pancreaticoduodenal vein and duodenal congestion lesions, which were completely counteracted by BPC 157 application, both with intraabdominal bath or intragastric application with rapid presentation of the bypassing pathway through inferior anterior pancreaticoduodenal vein to superior mesenteric vein. This corresponds to the general evidence that BPC 157 was used in ulcerative colitis trial and duodenal lesions counteraction. As a proof of the integrative healing evidence (rapid cytoprotective endothelium rescue that BPC 157 exerted may be useful against damaging chain of events during ischemia [two ligations] and during reperfusion [ligations removed]) appear normalized NO- and malondialdehyde (MDA)-values in colon tissues, oxidative stress markers. On the other hand, with ligation of the inferior caval vein occlusion we made a recapitulation of Virchow. A consistent model goes with ligation leading to vessel injury, exposure of tissue factors, stasis, thrombosis, hemodynamic changes, arterial hypotension, and in particular, venous hypertension, which approximates values over 20 mm Hg in the inferior caval vein, thereby being roughly four times higher than those in normal rats. All these adverse effects were counteracted by BPC 157 application. Interestingly, after cecum perforation, with BPC 157 administration we documented the gross reappearance of the vessels (USB micro camera) quickly propagating toward the defect at the caecum surface, defect in contraction, bleeding attenuation, MDA- and NO-levels normalized in colon tissue at 15 minutes, and advanced healing of colon lesions and less adhesions at days 1 and 7.
tetrahydrophyridine (MPTP; Parkinson’s model in mice), reserpine, haloperidol and other dopamine antagonists. Thus, BPC 157 does not act as a serotonin substrate. BPC 157 does inhibit MAO-inhibition, modulates serotonergic and dopaminergic systems, beneficially affects various behavioral disturbances that otherwise appeared due to specifically (over)stimulated/damaged neurotransmitters systems. (1)
⦁ Sikiric, Predrag et al. “Stable Gastric Pentadecapeptide BPC 157, Robert’s Stomach Cytoprotection/Adaptive Cytoprotection/Organoprotection, and Selye’s Stress Coping Response: Progress, Achievements, and the Future.” Gut and liver vol. 14,2 (2020):
153-167. doi:10.5009/gnl18490
⦁ Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, Sever M, Klicek R, Radic B, Drmic D, Ilic S, Kolenc D, Vrcic H, Sebecic B. Stable gastric pentadecapeptide BPC 157: novel therapy in gastrointestinal tract. Curr Pharm Des. 2011;17(16):1612-32. doi: ⦁ 10.2174/138161211796196954. PMID: 21548867.
⦁ redrag Sikirić, Sven Seiwerth, Željko Grabarević, Rudolf Ručman, Marijan Petek, Vjekoslav Jagić, Branko Turković, Ivo Rotkvić, Stjepan Miše, Ivan Zoričić, Paško Konjevoda, Darko Perović, Ljubica Jurina, Jadranka Šeparović, Miro Hanževački, Branka Artuković, Mirna Bratulić, Marina Tišljar, Miro Gjurašin, Pavao Miklić, Dinko Stančić-Rokotov, Zoran Slobodnjak, Nikola Jelovac, Anton Marović,
The influence of a novel pentadecapeptide, BPC 157, on NG-nitro-l-arginine methylester and l-arginine effects on stomach mucosa integrity and blood pressure,
European Journal of Pharmacology,
Volume 332, Issue 1, 1997, Pages 23-33, ISSN 0014-2999, https://doi.org/10.1016/S0014-2999(97)01033-9
⦁ Seiwerth S, Rucman R, Turkovic B, Sever M, Klicek R, Radic B, Drmic D, Stupnisek M, Misic M, Vuletic LB, Pavlov KH, Barisic I, Kokot A, Japjec M, Blagaic AB, Tvrdeic A, Rokotov DS, Vrcic H, Staresinic M, Sebecic B, Sikiric P. BPC 157 and Standard Angiogenic Growth Factors. Gastrointestinal Tract Healing, Lessons from Tendon, Ligament, Muscle and Bone Healing. Curr Pharm Des. 2018;24(18):1972-1989. DOI: ⦁ 10.2174/1381612824666180712110447. PMID: 29998800.
Stable BPC-157 (Arginate Salt) 250MCG/Capsule
$99.90