Adipotide-FTPP 10mg
$84.90
This product is intended for research and medical purposes only, to be used by trained professionals.
Research has suggested MOTS-c is an exercise mimetic, a 16-amino-acid peptide that is encoded in the mitochondrial genome of cells. According to clinical studies, it improves insulin sensitivity. While the study is still ongoing, control group examinations using diet-induced obese mice have shown improvements in insulin sensitivity. Researchers are still studying to see if the MOTS-c changes plasma markers, which are used to determine the body’s metabolic condition.
Research has shown that it targets body muscles and regulates its metabolism using the folate-purine-AMPK pathway. By doing so, it becomes effective protection against obesity and insulin resistance that stems from the diet that one is taking or age.
HOW MOTS-C PEPTIDE REGULATES GLUCOSE
Clinical studies have shown that MOTS-c promotes the production of endogen AMP analogue AICAR, which in turn, helps in the synthesis of AMP-activated protein kinase [1] (also referred to as AMPK). Before the production of AMPK, its cellular actions inhibit the folate cycle along with de novo purine biosynthesis. The AMPK then induces systemic cellular uptake of glucose and enhances insulin sensitivity. These studies suggest the mitochondria [2] offer active regulation of metabolic homeostasis via this peptide encoded in their genome.
Research has confirmed that the peptide genome can help reduce insulin resistance [3]. Health experts recommend taking the medication along with a strict, balanced diet to lower the amount of fat taken to manage obesity. Other studies have shown that general weight gain may increase insulin insensitivity, especially on diabetes type 1 patients. Cutting down the fat intake and increased levels of MOTS-c may lower the diet-induced metabolic dysfunction and associated inflammation.
References:
[1] https://physoc.onlinelibrary.wiley.com/doi/full/10.14814/phy2.14171
- Buy 5 and save 10%
- Buy 10 and save 15%
Legal Notice Regarding This Product
THIS PRODUCT IS STRICTLY INTENDED FOR RESEARCH USE ONLY. It is designated solely as a research chemical. Usage is confined to in vitro testing and laboratory experimentation exclusively. Information available on our website about this product is for educational purposes only and should not be interpreted as an endorsement or directive for use outside these bounds. Introducing this product into humans or animals by any route is strictly prohibited by law. Handling of this product should be undertaken only by licensed, qualified professionals.
This product is not to be used as a drug, food, or cosmetic. Misbranding, misusing, or mislabeling this product as a drug, food, or cosmetic is illegal. Users are responsible for complying with all applicable laws and regulations regarding the handling and use of this product.
Batch:BP3499C
| Dates |
19 June 2024
Avg Mass:
10 mg
|
|---|---|
| Janoshik Lab Results |
14.43 mg
Full Report:
|
| MZ Biolabs Lab Results | 99.866% |
THE PRODUCTS PURCHASED ON THIS WEBSITE ARE INTENDED FOR RESEARCH CHEMICAL USE ONLY. These products are not intended to be used for human or animal consumption and/or ingestion of any kind. These products should not be used as food additives, drugs or household chemicals. These products should only be used by Qualified Professionals.
Bodily introduction into Human and Animals of any kind is strictly forbidden by law.
All the product information on this website is for educational purpose only.
ADIPOTIDE
Product Usage: THE PRODUCT IS INTENDED AS A RESEARCH PURPOSE ONLY. This designation allows the use of research chemical strictly for in vitro testing and laboratory experimentation only. All product information available on www.peptidesworld.com is for educational purposes only. Bodily introduction of any food, cosmetic and may not be misbranded, misused or mislabeled as a drug, food or cosmetics.
HIGHLIGHTS:
- Adipotide starving fat cells.
- Weight loss in a study wita obese rhesus macaques.
- After four weeks of daily injections of adipotide and a four-week follow-up with no treatment — and without forced changes in diet or exercise — 10 obese female rhesus macaques lost an average of 11% of body weight and 39% of fat deposits.
- Adipotide as Cancer treatment
Obesity, defined as body mass index greater than 30, is a leading cause of morbidity and mortality and a financial burden worldwide. Despite significant efforts in the past decade, very few drugs have been successfully developed for the treatment of obese patients. Biological differences between rodents and primates are a major hurdle for translation of anti-obesity strategies either discovered or developed in rodents into effective human therapeutics. Here, we evaluate the ligand-directed peptidomimetic CKGGRAKDC-GG-D(KLAKLAK)2 (henceforth termed adipotide) in obese Old World monkeys. Treatment with adipotide induced targeted apoptosis within blood vessels of white adipose tissue and resulted in rapid weight loss and improved insulin resistance in obese monkeys. Magnetic resonance imaging and dual-energy x-ray absorptiometry confirmed a marked reduction in white adipose tissue. At experimentally determined optimal doses, monkeys from three different species displayed predictable and reversible changes in renal proximal tubule function. Together, these data in primates establish adipotide as a prototype in a new class of candidate drugs that may be useful for treating obesity in humans. [1]
We first used spontaneously obese rhesus macaques (Macaca mulatta), one of the most relevant primate models for human obesity and its associated comorbidities. In an initial dose-finding study, female rhesus macaques (n = 4) aged 9 to 13 years were selected for study entry on the basis of a history of spontaneous obesity, increased food consumption, and failure to maintain a high level of activity. All animals were defined as obese by a body mass index (BMI) ranging from 34 to 45 according to the equivalent formula for rhesus macaques (31). Body weights ranged from 10.0 to 11.7 kg, and the heaviest monkey had a morbidly obese body habitus (BMI = 45). All monkeys had the most clinically abundant abdominal fat in a central pattern relative to that of a large cohort of adult females (n = 350) in the breeding colony. Individual monkeys were euglycemic, with fasting serum glucose levels ranging from 65 to 71 mg/dl (normal range, <80 mg/dl) and serum insulin levels ranging from 21 to 52 μU/ml (normal range, <41 μU/ml). Adipotide was prepared under Good Manufacturing Practice (GMP) to our specifications by PolyPeptide Laboratories. Daily subcutaneous treatment with increasing doses of adipotide resulted in a dose-dependent decrease in body weight, BMI, and abdominal circumference relative to that of negative control monkeys receiving saline (Fig. 1, A to C). After dosing for 9 weeks, the body weight of the two treated monkeys in this initial cohort decreased by 1.8 kg (15.4%) and 2.1 kg (20.4%; Fig. 1A). Moreover, the BMI decreased from 45.0 to 37.3 (17.1%) in the morbidly obese monkey and from 38.8 to 30.9 (20.4%) in the second treated monkey (Fig. 1B). Finally, abdominal circumferences were reduced by 6.5 cm (13.1%) and 9.0 cm (14.2%) from baseline (pretreatment) measurements (Fig. 1C). During the same time period, the body weight, BMI, and abdominal circumference of the obese control monkeys receiving only saline did not change. [1]
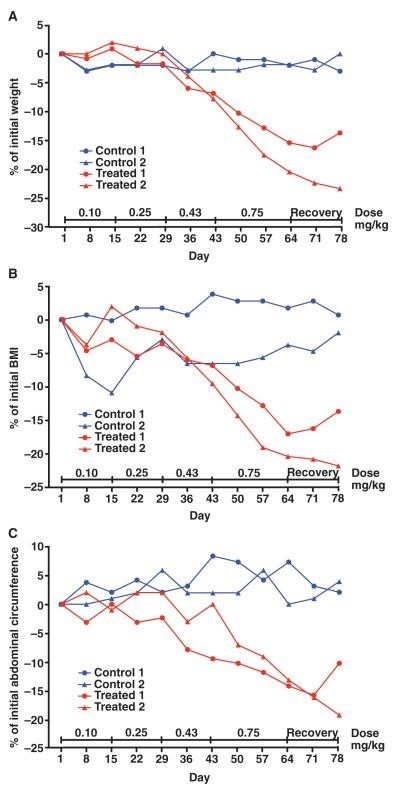
FIG 1 Anthropometric assessment of four obese rhesus monkeys treated with increasing doses of adipotide (0.10, 0.25, 0.43, and 0.75 mg/kg). (A to C) Obese rhesus monkeys displayed improved anthropometric measurements in a time- and dose-dependent manner. The percentage change from baseline for body weight (A), BMI (B), and abdominal circumference (C) was calculated weekly throughout dosing and recovery. Dose-dependent decreases occurred for these parameters in obese monkeys receiving adipotide; in contrast, no changes were observed in monkeys receiving saline. After a 2-week recovery period, the decrease in body weight, abdominal circumference, and BMI began to reverse. [1]
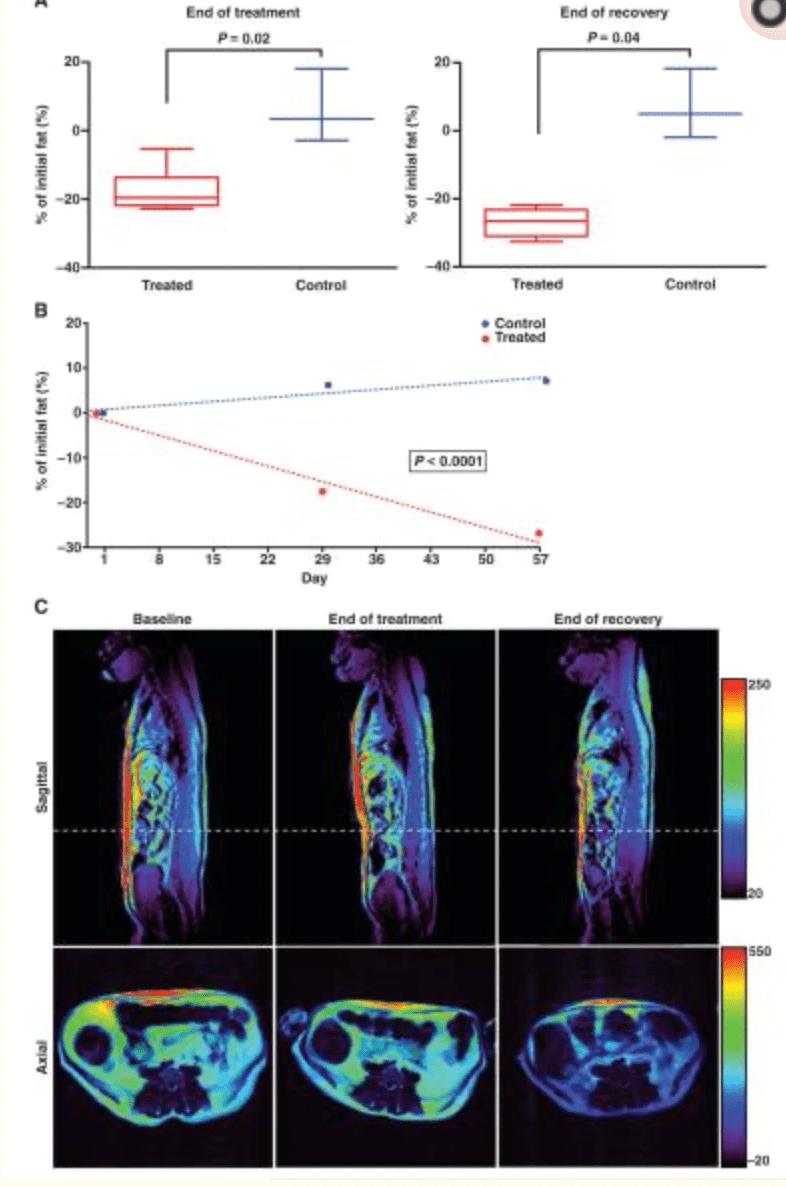
FIG 2
MRI confirms that weight loss results from a marked decrease in the volume of white adipose tissue. (A) The percentage change in fat volume was determined by quantifying the volume with axial T1-weighted MR images. The change is represented as percentage change from baseline (day 1) and is significantly decreased at the end of treatment and at the end of recovery (Mann-Whitney-Wilcoxon test, P = 0.02 and P = 0.04, respectively). Error bars indicate SEM (control, n = 3; treated, n = 6). (B) A mixed-effects model of the data over time indicates significance in the decreasing fat percentage for the treated versus control groups (P < 0.0001). (C) Representative sagittal and axial T1-weighted images from one of the treated animals. The window level range is indicated by the color bar on the right. Axial images are taken at the cross section indicated by the white dashed line in the sagittal image. A decrease in fat content is represented by a decrease in window level (that is, intensity of the image display).
Cancer treatment shows promise for rapid weight loss
“Cancer treatment shows promise for rapid weight loss,” Randy Seeley of the University of Cincinnati College of Medicine: https://www.latimes.com/local/la-xpm-2011-nov-10-la-he-drug-fat-loss-20111110-story.html
Toxicology Studies
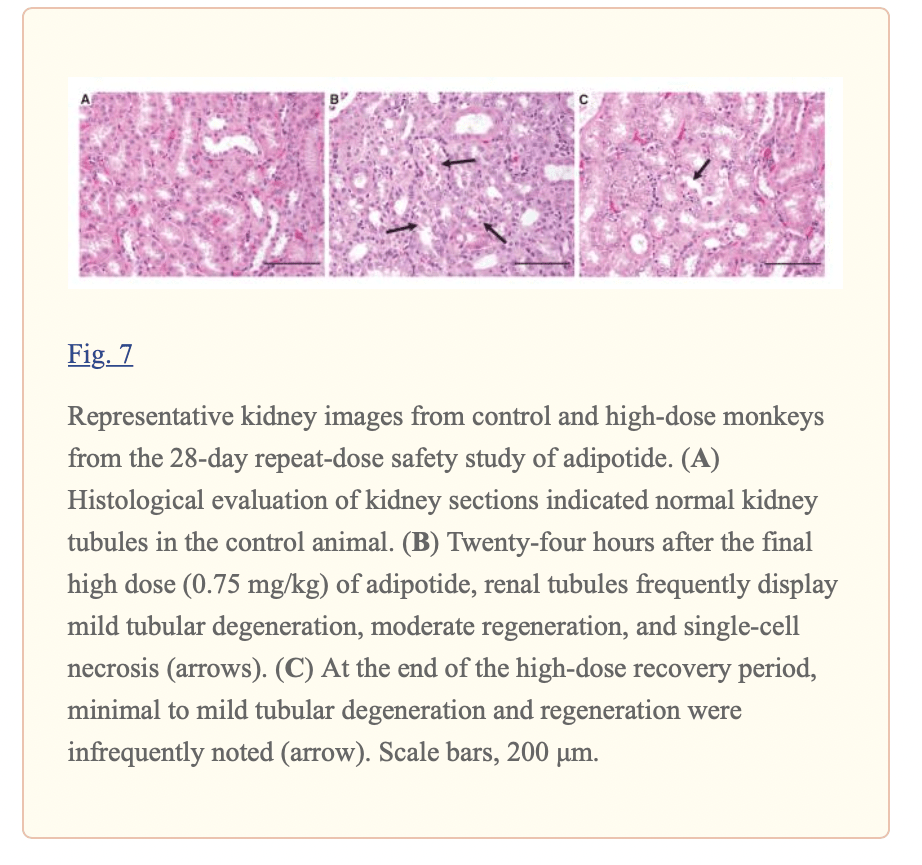
Barnhart, Kirstin F et al. “A peptidomimetic targeting white fat causes weight loss and improved insulin resistance in obese monkeys.” Science translational medicine vol. 3,108 (2011): 108ra112. doi:10.1126/scitranslmed.3002621

Adipotide-FTPP 10mg
$84.90